SCROLL
DOWN
As part of the agency's Leafy Greens STEC Action Plan, the U.S. Food and Drug Administration (FDA) has published a report detailing the results of targeted inspections and microbiological testing of leafy greens grown in Salinas Valley, California during the region's 2022 harvest season. The inspections, carried out by the California Department of Food and Agriculture (CDFA), and the sampling, done by FDA, included 14 farms that had been potentially linked to unresolved outbreaks of foodborne illness during traceback investigations in 2020 and 2021.
The aims of the inspections and testing were to identify potential contamination of leafy greens and to prevent contaminated or potentially contaminated product from entering commerce. The surveillance effort and related follow-up actions did prevent contaminated leafy greens from entering commerce, but did not find additional evidence to link any specific farms to the foodborne illness outbreaks in 2020 and 2021. All samples collected during the 2022 work in the Salinas Valley region were tested for Escherichia coli O157:H7 and Salmonella, as FDA detected non-O157 E. coli and Salmonella during its 2021 assignment following the outbreaks linked to lettuce grown in the region. Those findings, considered alongside the outbreaks, indicated to FDA that further surveillance was warranted, but more focused on farms potentially linked to the outbreaks.
Of the total 62 samples of leafy greens tested, FDA detected S. Enteritidis in one sample of romaine lettuce. The agency performed whole genome sequencing (WGS) analysis on the Salmonella recovered from the positive sample and found the bacteria to be closely related to human clinical and chicken isolates. However, FDA did not have sufficient epidemiological evidence to link the farm at which the sample was collected to clinical illnesses. Sediment samples were negative for each of the target pathogens.
Upon identifying S. enterica subspecies diarizone in a sample of green leaf lettuce, FDA notified the grower, shipper, and cooler about the positive sample. The farm destroyed the lot associated with the positive finding, and none of the potentially contaminated lettuce entered commerce. The identified subspecies of Salmonella was not found to have any clinical illness matches. At FDA's request, state partners initiated an on-farm inspection during the 2022 harvest season. This inspection included an assessment of animal intrusion, soil amendments, adjacent land use, and water use.
Additionally, FDA identified E. coli O157:H7 in a sample of iceberg lettuce, at which point FDA notified the grower, the harvester, and the owner/shipper/cooler about the positive sample and that the isolate was undergoing further characterization. The owner/shipper/cooler informed the agency that the entire lot of lettuce associated with the positive sample had been destroyed and none of it entered commerce. FDA and state partners initiated a follow-up investigation at the farm identified as the grower of the contaminated iceberg lettuce sample to determine potential sources and routes of contamination, but the investigation was limited because the field where the sampled lot of iceberg lettuce was harvested was fallow. All samples collected during the follow-up investigation tested negative for E. coli O157:H7 and other strains of STEC.

FDA Publishes Report on Investigations, Sampling of Leafy Greens in Salinas Valley
Scientists Develop Test That Detects PFAS in Under Three Minutes
Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images

Researchers from the New Jersey Institute of Technology (NJIT) have developed a method of detecting toxic per- and poly-fluoroalkyl substances (PFAS) in food packaging, water, and soil samples in three minutes or less. Existing tests can take hours for sample preparation and analysis. The new method leverages paper spray mass spectrometry (PS-MS)—an ionization technique for analyzing the molecular composition of sample materials—which is 10–100 times more sensitive than liquid chromatography/mass spectrometry, the current standard for PFAS testing.
PFAS can be ionized and rapidly detected by a high-resolution mass spectrometer, which gives a clear view of each type of PFAS present and the degree of contamination, down to a parts-per-trillion (ppt) level. For more complex matrices like soil, the researchers applied a related method called desalting paper spray mass spectrometry (DPS-MS), which washes away salts that normally suppress the ion signal of PFAS. Together, the two techniques greatly improve the ability to detect PFAS. The new method's limit of detection for PFAS is 1 ppt, which is equivalent to a single drop of water in 20 Olympic-sized swimming pools.
When testing the method, the researchers were able to detect PFAS in one minute or less in samples of microwave popcorn paper, instant noodle boxes, fast food wrappers, and other food packaging samples. The analysis revealed traces of 11 different PFAS molecules, including common types that have been linked to cancer and immune system suppression; specifically, perfluorooctanoic acid (PFOA) and perfluorooctanesulfonic acid (PFOS), both of which have been targeted by the U.S. Environmental Protection Agency (EPA) in a proposal to establish maximum contamination levels for six types of PFAS in drinking water.
When analyzing water, the researchers found traces of PFOA in tap water samples in under two minutes, while finding no traces of PFAS in samples taken from the university's filtered fountain water. Using DPS-MS, the team also identified two species of PFAS from as little as 40 milligrams of soil in under three minutes.
Using whole genome sequencing, the U.S. Centers for Disease Control and Prevention (CDC), FDA, and public health and regulatory officials in several states have solved a multistate foodborne illness outbreak investigation that has been ongoing since 2014, with the most recent illnesses being reported in December 2023. CDC first investigated this outbreak of illnesses caused by Listeria monocytogenes in 2017, and again in 2021. Epidemiologic evidence in previous investigations identified queso fresco and other similar cheeses as a potential source of the outbreak, but there was not enough information to identify a specific brand. CDC reopened the investigation in January 2024 after new illnesses were reported in December 2023 and the outbreak strain was found in a cheese sample from Rizo-López Foods in Hawaii.
Using CDC's PulseNet system, which houses a national database of foodborne pathogen DNA fingerprints identified through WGS, public health investigators found L. monocytogenes isolated from patients in 2014 to be closely related to isolates from more recent patients. The genetic similarity suggests that the people sickened in 2014–2023 were infected by the same food. During routine sampling in January 2024, the Hawaii State Department of Health's Food and Drug Branch collected a sample of aged cotija cheese product made by Rizo-López Foods, which tested positive for the strain of L. monocytogenes responsible for the outbreak. Subsequently, FDA conducted inspections at the Rizo-López Foods facility and collected food and environmental samples for testing. FDA found the outbreak strain of L. monocytogenes on a container where cheeses are kept before they are packaged.
As of February 6, 2024, a total of 26 people infected with the outbreak strain of L. monocytogenes have been reported from 11 states, resulting in 23 hospitalizations and two deaths. The onset of illnesses ranges from June 15, 2014–December 10, 2023. FDA has released a list of retail establishments that received Rizo-López Foods dairy products that includes over 100 retailers across seven states. FDA also continues to update its table of recalled products.

WGS Helps Solve a Decade-Long Listeria Outbreak Linked to Cheese
FDA Releases New Food Fraud Webpage
The U.S. Food and Drug Administration (FDA) has released a new website on economically motivated adulteration (EMA), including food fraud. The purpose of the website is to keep businesses and consumers informed on the latest food fraud developments.
The website includes links on how to report food fraud; examples of food adulteration; how food fraud is detected and monitored; enforcement and legal consequences, such as recalls, seizures, and import refusals; guidance documents to assist manufacturers and importers; and a list of import alerts.
EMA occurs when "someone intentionally leaves out, takes out, or substitutes a valuable ingredient or part of a food," according to FDA. EMA also occurs when a substance is added to a food to make it appear better or of greater value.
Food fraud is a common type of EMA that FDA investigates, but EMA also occurs with other products, including animal food and cosmetics. Some types of EMA are also misbranding violations. Estimating how frequently food fraud occurs or its exact economic impact can be challenging because food fraud is designed to avoid detection. Outside estimates by experts have found that food fraud affects 1 percent of the global food industry at a cost of approximately $10–$15 billion per year, although more recent expert estimates peg the cost as high as $40 billion per year.
Food fraud can also lead to major health issues and even death. Some examples include lead poisoning from adulterated spices and allergic reactions to a hidden or substituted ingredient that contains a small amount of just one food allergen.
Click here to visit FDA’s new EMA website.

Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images
In January 2024, a report written by the National Advisory Committee on Microbiological Criteria for Foods (NACMCF), titled, "Response to Questions Posed by the Food Safety and Inspection Service: Enhancing Salmonella Control in Poultry Products," was published in the Journal of Food Protection. In the report, NACMCF provides scientific advice to federal food safety agencies, including the U.S. Department of Agriculture's Food Safety and Inspection Service (FSIS).
In light of FSIS' ongoing efforts to reduce Salmonella infections attributable to poultry, which include a new regulatory framework that was proposed in October 2022 , the purpose of the newly published report from NACMCF is to provide guidance to FSIS and the poultry industry on types of microbiological criteria that might be used to identify and incentivize effective pre- and postharvest Salmonella intervention strategies. The report takes into consideration scientific evidence on Salmonella control in the U.S. and abroad, foodborne illness surveillance data, quantitative microbial risk assessments, and microbiological testing of indicator organisms versus Salmonella on poultry throughout the farm‐to‐fork continuum.
The Landscape of Salmonella in Poultry
According to the report, the infectious dose of Salmonella varies widely between serotypes, with recent data suggesting that most poultry-associated outbreaks in the U.S. involve S. Enteritidis, S. Typhimurium, S. I:4,5,12:i:‐, S. Infantis, and S. Heidelberg. Furthermore, four of the five aforementioned serotypes (all excluding S. Heidelberg) account for 83 percent of chicken‐associated illnesses in the U.S. Vaccination against specific serotypes, such as S. Typhimurium, are common among U.S. broiler breeders, a strategy which has reduced the incidence of contamination of that serotype. However, vaccine development takes years and lags behind the shifting of predominant serotypes found in flocks. Other U.S. preharvest management practices include competitive exclusion, controlling the quality of feed, biosecurity, moisture control in poultry houses, and clean transport coops.
The relative number of salmonellosis cases in the U.S. caused by S. Typhimurium and S. Heidelberg has declined during the past 20 years, likely due in part to the commercial poultry vaccine used against S. Typhimurium also delivering cross‐protection against S. Heidelberg. Although progress has been made against these two serotypes, overall cases of salmonellosis attributed to poultry remain unchanged, suggesting that it may be necessary to develop alternate methods for controlling and detecting Salmonella that do not rely solely on serotype. Furthermore, attribution data does not specifically identify whether sources of Salmonella were whole carcasses, parts, comminuted product, or breaded raw poultry products. NACMCF underlines that more granular data will help determine if all poultry products pose the same risk and allow a targeted management program.
Qualitative testing for total Salmonella at breeder or broiler farms can be focused on environmental samples and cecal testing, but sufficiently sensitive tests and specific serotype testing are needed to determine if any changes are required to control Salmonella serovars that are most often associated with human illness. NACMCF suggests targeting highly contaminated birds for logistic slaughter (i.e., scheduling their slaughter after less contaminated flocks) or other interventions, based on results from microbial testing at farms.
Although indicator organisms such as Enterobacteriaceae or aerobic plate counts (APCs) have been used by industry to gauge the efficacy of process control and to measure microbial reduction on carcasses from slaughter to post-chill, some studies have shown that populations of these indicators are not directly correlated to populations of Salmonella. In light of the "conflicting and weak" correlation between the presence or levels of indicator organisms and that of Salmonella post-carcass wash, NACMCF suggests basing microbiological criteria on Salmonella enumeration.
Moreover, microbial risk assessments have shown that diverting ground turkey product that tests above a set threshold of Salmonella colony forming units (CFU) per gram, compared to current protocols (i.e. not diverting), is expected to remove product from the market that has higher chances of causing illness. Such a threshold would need to be clearly linked to health‐based targets. This concept is currently used by industry whereby poultry used in breaded and stuffed raw chicken product are enumerated for Salmonella by quantitative polymerase chain reaction (qPCR), not by targeting specific serotypes.
NACMCF Recommendations to FSIS
Key components of FSIS' proposed regulatory framework include requiring that incoming flocks be tested for Salmonella before entering an establishment, that establishments enhance process control monitoring, and that the agency implements an enforceable final product standard. Current performance standards include all Salmonella serotypes, rather than quantification of specific highly pathogenic serotypes. NACMCF believes that an approach targeting highly pathogenic serotypes could trigger additional mitigating actions and could be more effective in diverting products that have higher infectious potential. The public health benefits of such an approach should be evaluated through a comprehensive quantitative risk assessment.
NACMCF identified a multitude of data gaps that could affect findings and recommendations to FSIS, including the need for completion of the two quantitative risk assessments for chicken and turkey, which were in progress at the time of the report's completion. According to a summary of FSIS achievements that was published in January 2024, the quantitative risk assessments for Salmonella in chicken and turkey were completed in 2023.
NACMCF's recommendations to FSIS, some of which FSIS may have already progressed, include:
- Collecting appropriate data to refine food attribution models and determine which form(s) of raw poultry exposure (e.g., consuming processed, parts, whole carcasses, handling live poultry, exposure to poultry manure, etc.) and food handler practices contribute most to salmonellosis associated with chicken and turkey
- Expanding systematic sampling for Salmonella levels, prevalence, and serotypes on poultry preharvest (hatcheries, feed, poultry houses) and FSIS postharvest sampling (slaughter through processing), prioritizing product lines that historically are more frequently contaminated (those that are not further processed using a validated lethality step and have been linked to illness, such as comminuted poultry products, chicken parts/pieces, and breaded stuffed raw chicken products)
- Incentivize industry to deposit anonymous, nonpunitive data on levels of indicator organisms and Salmonella prevalence, concentration, and serotypes found at various stages of processing, along with practices that may mitigate contamination, and analyze this data to identify alternate process control indicators, to use in risk assessments to update performance standards, and to determine how non‐Salmonella quality indicator sampling could be established for targeting flock houses with a higher probability of contamination
- Every 2–3 years, compare serotypes isolated from salmonellosis patients with those isolated from poultry products to determine if intervention strategies used by industry are effective against all Salmonella serotypes or are selecting for specific serotypes
- Develop and validate quantitative testing methods to determine if and how testing and processing scheduling can reduce the likelihood that carcasses and parts with higher levels of Salmonella that are most capable of causing illness are released into commerce
- Complete risk assessments for chicken and turkey to assess public health impacts of different risk‐based Salmonella control strategies, including qualitative and quantitative performance standards, possibly complemented by serotype identification
- Upon completion of the risk assessments, consider developing changes to performance standards based on the findings
- Incentivize industry to develop, validate, and universally implement robust Salmonella mitigation programs and qualitative Salmonella testing at the breeder, hatchery, grow-out, and transport levels; targeting for conditions in houses, transport crates, and holding areas that harbor and transmit Salmonella by universal implementation of known and validated mitigation strategies
- Reevaluate NACMCF's report and suggestions within 3–5 years, after appropriate data have been collected and risk assessments are complete, addressing the gaps identified by the committee.

NACMCF Reports on Reducing Salmonella in Poultry, Advises FSIS
The U.S. Department of Agriculture's Agricultural Marketing Service (USDA AMS) published its 2022 Pesticide Data Program (PDP) Annual Summary on January 30, 2024, showing that over 99 percent of samples tested had pesticide residues below the Environmental Protection Agency's (EPA's) established benchmark levels. Additionally, 27.6 percent of samples had no detectable residue. However, testing for persistent environmental contaminants that are no longer used as pesticides in the U.S. showed the presence of certain banned chemicals at varying levels in some food commodities.
USDA AMS tested a total of 10,665 samples from 23 commodities including fresh and processed vegetables and fruits, grains, nuts, and dairy. To collect PDP data, USDA works with state agencies representing all census regions of the country and nearly half of the U.S. population. In 2022, analyzed samples were collected in California, Colorado, Florida, Maryland, Michigan, New York, Ohio, Texas, and Washington.
Since PDP data are used for risk assessments, PDP laboratory methods are geared to detect very low levels of pesticide residues, even when those levels are well below the tolerances established by EPA. Prior to testing, PDP analysts washed samples for 15–20 seconds with gently running, cold water as a consumer might do; no chemicals, soaps, or any special washes were used.
Residues exceeding EPA tolerances were detected in 56 (0.53 percent) of the total 10,665 samples tested. Of these 56 samples, 19 were domestic (33.9 percent) and 37 were imported (66.1 percent). Residues with no established tolerance were found in 2.5 percent (269 samples) of the total samples tested, 127 of which were domestic (47.2 percent) and 142 of which were imported (52.8 percent).
Fresh and processed fruit and vegetables accounted for 8,512 samples (79.8 percent) of the total 10,665 samples collected in 2022. Fresh and processed fruit and vegetables tested were: baby food green beans, baby food peaches, baby food pears, baby food sweet potatoes, blueberries (fresh and frozen), carrots, celery, grapes, green beans, mushrooms, peaches (fresh and frozen), pears, plums, potatoes, summer squash, tomatoes, and watermelon.
PDP laboratories also test foods for low levels of environmental contaminants that are no longer used as pesticides in the U.S., but due to their persistence in the environment, can be taken up by plants. For example, use of DDT has been prohibited in the U.S. since 1972; however, due to its persistence in the environment, low-level residues of DDT and its DDE metabolite were detected in some commodities tested. The DDE metabolite was the most frequently detected, being found in butter (35.4 percent of samples), summer squash (5.2 percent), potatoes (2.3 percent), celery (1.4 percent), and green beans (0.6 percent). DDT was detected in summer squash (4.5 percent), and DDT p,p' was detected in summer squash (3.4 percent) and potatoes and plums (0.2 percent each).
Other chemicals that have been banned for use in the U.S. for more than 30 years but were still present in food samples included chlordane (cis and trans), dieldrin, endrin, and heptachlor (epoxide). Chlordane cis was detected in 2.6 percent of summer squash samples, and chlordane trans was detected in 2.2 percent of summer squash samples. Dieldrin was detected in 3.8 percent of summer squash samples and 0.2 percent of butter samples. Endrin was detected in 1.3 percent of summer squash samples and 0.1 percent of watermelon samples. Heptachlor epoxide was detected in 3.2 percent of summer squash samples.
No residues of aldrin, BHC (alpha/beta/delta/epsilon), HCB, heptachlor (parent), lindane (BHC gamma), or mirex were detected in any samples.

USDA Finds 99 Percent of Pesticide Residue Samples Below Benchmarks
FDA has released the CORE 2022 Annual Report—the first report of its kind—summarizing the investigations of foodborne illness outbreaks and adverse events involving FDA-regulated foods conducted by the Coordinated Outbreak Response and Evaluation (CORE) Network.
Founded in 2011, the mission of CORE is to find, stop, and aid in the prevention of foodborne illness outbreaks, in collaboration with CDC and state and local partners. The CORE Network provides a publicly available investigation table that is updated weekly and provides information about ongoing foodborne illness outbreak and adverse event investigations. CORE does not cover seafood-related illnesses or incidents related to animal feed or pet food, which are handled by different FDA departments.
In 2022, CORE evaluated, responded to, and issued advisories for 65, 28, and 11 incidents, respectively. This is a slight increase over 2021, which saw 59 evaluated incidents, 19 responses, and 11 advisories issued. However, 2022 saw the third-lowest number of incidents evaluated in CORE's ten years of operation. Of the incident responses with identified products linked to illness, produce was associated with five incidents (37 percent), other or unknown commodities were associated with four incidents (31 percent), and nuts and seeds, fish, dairy, and multi-ingredient foods were linked to one incident each (8 percent each). Specific food products that were implicated include enoki mushrooms, alfalfa sprouts, cantaloupe, strawberries, romaine lettuce, ice cream, Brie and Camembert cheese, multiple types of fish, peanut better, plant-based crumbles, a meal replacement drink, powdered infant formula, and falafel.
FDA took several actions based on CORE investigations in 2022, the most notable being the commodity-specific prevention strategy for Cronobacter sakazakii contamination of powdered infant formula, following a prolific recall that led to a national shortage of formula. Aside from the prevention strategy, FDA issued 11 advisories, nine recall announcements, and one nationwide import alert.
Other noteworthy activity included two outbreaks linked to enoki mushrooms that took place in 2020 and 2022, resulting in more than 25 recalls due to potential Listeria monocytogenes contamination as of August 2023. The 2020 outbreak and subsequent increase in surveillance prompted the release of a commodity-specific prevention strategy for L. monocytogenes contamination of enoki and wood ear mushrooms. Additionally, after the 2022 outbreak, FDA added enoki mushrooms from China to an import alert that was originally established for enoki mushrooms imported from the Republic of Korea based on information gathered during and following the 2020 outbreak.
Another important event was an August 2022 multistate outbreak of Salmonella Typhimurium linked to cantaloupe. The isolates in this cluster of illnesses were within seven alleles and 11 single-nucleotide polymorphisms (SNPs) of two FDA soil swab samples collected from a 2020 outbreak investigation in Indiana. Based on traceback information from the 2022 outbreak, FDA conducted investigations in Indiana at three farms, their common packinghouse, and nearby public lands. Salmonella-positive environmental samples were found at each location, but none of the resulting Salmonella isolates conclusively matched the outbreak strain by whole genome sequencing (WGS). The outbreak vehicle was confirmed after the outbreak ended. No cantaloupes were recalled, and no public warning was issued as the implicated products were no longer on the market.
The last significant outbreak highlighted by FDA in the report is a multistate outbreak of Salmonella Senftenberg infections linked to certain peanut butter products, for which voluntary recalls and a warning letter were issued. FDA is preparing future communications to discuss findings and provide information to assist in future prevention efforts.
The report highlighted the use of the Center for Food Safety and Applied Nutrition (CFSAN) Adverse Event Reporting System (CAERS), a way for consumers and healthcare providers to voluntarily submit complaints and data, which led to the investigation of four incidents involving powdered infant formula, a meal replacement drink, dry cereal, and frozen food. These types of investigations come with unique challenges, such as not always having all of the necessary data and lacking laboratory testing information. Notably, the report mentions that in 2022, to handle the increasing workload of investigating outbreaks and adverse events, CORE established a permanent fourth Response Team with experts dedicated to solving and stopping outbreaks.
Finally, the report acknowledged the publications it issued during 2022 to communicate its activities, findings, and knowledge with the public. A total of 42 publications were put out in 2020–2022, tripling the average annual publication efforts during the 2012–2019 time period. Both of the trade press magazine articles published by CORE in 2022 were published with Food Safety Magazine, specifically, "The Incident Command System and Foodborne Illness Outbreak Investigations" in the October/November 2022 issue, and "Outbreak Investigations of Cyclospora cayetanensis Infections 2013–2020: Progress Made and Challenges Remaining" in the April/May 2022 issue.

FDA Publishes First CORE Annual Report Summarizing Outbreak Investigations
Scientists from USDA's Agricultural Research Service (USDA's ARS) have provided new insight into the ability of Salmonella to survive and adapt in food processing facilities through interactions with environmental biofilms. Unlike previous research that focuses primarily on single-species biofilms, the ARS project explores the more complex and realistic scenario where foodborne pathogens coexist with a multitude of environmental microorganisms in intricate, mixed biofilms.
The study reinforces that Salmonella's ability to interact in diverse biofilms improves its stress tolerance, lending to the pathogen's ability to colonize food contact surfaces, outcompete resident microorganisms, and resist sanitizers. The research represents the first step in explaining the science of real-world interactions between foodborne pathogens and biofilms, providing insights that can help inform enhanced food safety practices.
Although additional studies will be required to optimize food safety strategies informed by these findings, the groundbreaking research sheds light on the pivotal role of beneficial bacteria in the fight against foodborne pathogens. The study provides a new understanding of the role of environmental factors in shaping pathogen behavior, and shows the potential of targeting unique environmental species that can either protect or inhibit Salmonella, offering precise monitoring and intervention points.
The genetic diversity of Salmonella raises the question of whether the survival and adaptation strategies identified in the ARS study are applicable to other foodborne bacteria with similar diversity. Preliminary findings suggest that pathogens other than Salmonella may behave similarly when navigating the environment and interacting with other species. Ongoing research will explore how environmental microbiomes influence the tolerance and survival of various foodborne pathogens, as well as the association between environmental microbial communities and pathogen prevalence, the microbial ecology within meat processing environments, and the complex interactions within pathogen-environmental biofilms.

New Insights About Salmonella Interactions With Environmental Biofilms
Recent testing for toxic plasticizers in a wide range of food samples has revealed the pervasive presence of phthalates, often at high levels. At the same time, the study, conducted by Consumer Reports, also found a notable reduction in the levels of bisphenol A (BPA) and other bisphenols compared to 2009 (when Consumer Reports last tested for BPA in foods), although the chemicals are still broadly present in the U.S. food supply.
Phthalates and bisphenols like BPA are used in plastics and can be found in food packaging and other food contact materials, such as surfaces in food processing plants and food handling gloves. Their use has become an issue of concern due to increasing evidence pointing to the chemicals' negative health consequences, such as disruption of the endocrine system, tumor growth, abnormal reproductive function, neurological harm, immune issues, and other effects.
In April 2023, the European Food Safety Authority (EFSA) declared exposure to BPA to be a health concern after lowering the tolerable daily intake (TDI) for the plasticizer by 20,000—a level that most consumers exceed. In 2022, FDA also amended its food additive regulations, revoking the authorization for food contact use of 23 phthalates and two other substances. In the U.S., BPA is banned for use in baby bottles and infant formula cans.
The Study
For the study, Consumer Reports tested for BPA, bisphenol F, bisphenol S, ten different food-relevant phthalates, and three phthalate replacement compounds in 85 products purchased from supermarkets and fast food restaurants. A total of 239 samples were collected, including baked products and grains, beverages, condiments, fast foods, fruits and vegetables, infant foods, meat and poultry products, milk and other dairy products, seafood products, vegetable oils, and other commodities. The products' packaging types included aluminum foil, paper wrap, can, foam trays, plastic wrap, glass with lined lids, paper wraps, paper bags, cardboard, plastic bags, and pouches. The foods selected for sampling were chosen based on their likelihood to contain phthalates and bisphenols. Of the 85 total products tested, 67 were supermarket goods and 18 were fast foods, and 2–3 of each product was purchased for testing. The samples were collected between February and April 2023 from retailers in New York, New Jersey, and Connecticut.
After testing the samples, Consumer Reports averaged the sum of the levels of total phthalates and total bisphenols in the 2–3 samples of each food. A risk assessment for consumer exposure to phthalates and bisphenols was conducted based on estimates of U.S. adult intakes for the sampled foods, and the intake estimates were compared to EFSA, European Chemicals Agency (ECHA), and U.S. Environmental Protection Agency (EPA) TDIs for the chemicals, where possible.
The Findings
Consumer Reports found phthalates in nearly every food that was tested, often at high levels, and with no difference based on packaging type or food type. Especially high levels of the plasticizer were found in Del Monte sliced peaches, Chicken of the Sea pink salmon, Fairlife Core Power high-protein chocolate milkshakes, Yoplait Original French vanilla low-fat yogurt, and several fast foods, including Wendy's crispy chicken nuggets, a Chipotle chicken burrito, and a Burger King Whopper with cheese. Interestingly, organic foods had just as high levels of phthalates as non-organic products, and the highest phthalate levels were found in an organic food—Annie's Organic cheesy ravioli.
Some foods had lower phthalate levels than others, however. For example, Pizza Hut Original Cheese Pan Pizza had half the phthalate of comparable pizza from Little Caesars. Levels of phthalates even varied among foods from the same brand, such as Chef Boyardee's Big Bowl Beefaroni pasta in meat sauce, which had less than half the level of phthalates in the company's Beefaroni pasta in tomato and meat sauce. The only food sample in which phthalates were not detected was Polar raspberry lime seltzer.
Bisphenols were found in 79 percent of tested samples. Although the pervasiveness of bisphenols is still great, the levels of the chemicals were much lower than 15 years ago, when Consumer Reports last tested for BPA.

High Levels of Plasticizers Phthalates, Bisphenols Found in Nearly All Foods in U.S.

Smithfield Foods Inc. named Kraig Westerbeek as its new President of Hog Production Operations.
McLane announced the appointment of Terry Levee as its new Senior Director of Food Safety and Quality Assurance.
Pacteon Group appointed Janet Darnley as its new Vice President of Marketing.
The International Food Information Council (IFIC) appointed Milton Stokes, Ph.D., M.P.H., R.D., F.A.N.D., as IFIC Senior Director of Food and Nutrition.
The Alliance for Food and Farming (AFF) elected Ian LeMay, Incoming President of the California Table Grape Commission, as its new Chair.
The World Packaging Organization (WPO) announced new vice presidents to lead WPO's portfolios during 2024–2026: Vice President for Marketing Soha Atallah, Vice President for Sustainability and Save Food Nerida Kelton, Vice President for Education Kofi Essuman, and Vice President for Governance Magnus Sidling.
QualiTru welcomed Rikka Kerber and Sheila Berger to its team as new Strategic Account Executives.
The Partnership for Food Safety Education appointed four new board members: Jane DeMarchi, president of the North American Millers' Association; Stefanie Evans, Ph.D.; and H. Lester Schonberger, Ph.D., associate extension specialist with Virginia Tech/Virginia Cooperative Extension. Sharon Mayl, J.D., partner at DLA Piper, was appointed as board advisor.

WESTERBEEK

DARNLEY

STOKES

LEMAY
Jennifer McEntire, Ph.D., has joined Provision as Strategic Advisor and head of its new Strategic Advisory Group.
FDA, bioMérieux Partner to Improve Foodborne Pathogen Detection
bioMérieux has entered an agreement with FDA to collaboratively develop tools to combat foodborne pathogens, including improved detection and microbial characterization systems. The inaugural projects between bioMérieux and FDA will focus on using novel technologies to improve the isolation of Shiga toxin-producing E. coli (STEC), enhance detection of Cyclospora cayetanensis, and simplify microbial characterization methods for foodborne pathogens such as Salmonella and Listeria monocytogenes. The projects are being developed through bioMérieux's xPRO™ Program and FDA's Center for Food Safety and Applied Nutrition (CFSAN), Office of Applied Research and Safety Assessment (OARSA), and Office of Regulatory Science (ORS).

The SQF Institute (SQFI) intends to develop and release a new SQF edition, and has shared its process and proposed timeline for developing SQF Edition 10. The need for a new edition of the Safe Quality Food (SQF) standard is typically driven by various factors that may include changes in the industry landscape, advancements in food safety science, regulatory updates, data analysis, and feedback from stakeholders. SQFI typically engages in a systematic process, including stakeholder consultations and public reviews, to gather input before finalizing and releasing a new edition of the standard. A proposed timeline for SQF Edition 10 is as follows:
- 2024 Q1: Defining scope and goals, engaging stakeholder feedback, defining timeline and resources, and SQF Unites engagement
- 2024 Q2–Q4: Establishing technical working groups, creating the Codes, developing the implementation tools, and incorporating GFSI Benchmarking
- 2025 Q1–Q2: Release Edition 10 and establish communication plan
- 2025 Q3: Implementation period over a minimum of six months
- 2025 Q3–Q4: GFSI Benchmarking.
SQFI Developing New SQF Edition 10, Release Slated for 2025

Seafood traceability solutions providers Trace Register and Wholechain have achieved interoperability between their two different traceability systems based on Global Dialogue on Seafood Traceability (GDST) compatibility standards. These collaborative efforts will further help industry meet compliance with the requirements of the Seafood Import Monitoring Program (SIMP) and the Food Safety Modernization Act (FSMA) Food Traceability Final Rule. Working with GDST and other industry stakeholders, Trace Register and Wholechain collaborated in the development of the standards. The two competitors then implemented an extension of GDST standards using GS1's EPCIS capture interface to make GDST compatibility more practical between suppliers and buyers. The success of this development led GDST to incorporate the EPCIS capture interface method as an official supplement to its comprehensive standards.
The integration achieved through this collaboration facilitates a seamless two-way exchange of information. Notable examples include Trace Register customers shipping to a Wholechain customer, a major privately held supermarket chain in the U.S. Likewise, Wholechain customers can ship to a Trace Register client, another significant player in the supermarket industry. The seamless, automatic GDST data transmission eliminates the need for manual keying and safeguards against losing critical GDST data points. It also exemplifies the power of collaboration and interoperability in advancing seafood traceability.

First Seafood Traceability Providers Achieve Interoperability at Scale
A recent joint publication between FDA and Rheonix Inc. has confirmed the efficacy of an assay to detect Cyclospora cayetanensis, developed under a Research Collaboration Agreement (RCA) between Rheonix and FDA's Office of Applied Research and Safety Assessment (OARSA). The study demonstrated that the fully automated Rheonix C. cayetanensis™ assay achieves consistent detection rates for C. cayetanensis in samples of high-risk fresh produce with low levels of oocysts, including matrices such as herbs, leafy greens, and berries.
The Rheonix C. cayetanensis assay is based on genetic targets developed and published by FDA, and is processed using Rheonix reagents and consumables on the Rheonix Encompass Optimum™ workstation. The joint study shows the integration and verification of FDA's mitochondrial target into Rheonix's fully automated and streamlined workstation, simplifying workflow by performing DNA isolation, polymerase chain reaction (PCR), hybridization, results visualization, and reporting of results. The fully automated method enables detection of low levels of C. cayetanensis in produce samples, saving approximately four hours of hands-on effort per 24-sample run, in comparison to the prior workflow. The Rheonix assay is now available for use by food and environmental testing laboratories.

Automated Assay Developed by FDA, Rheonix Detects Low Levels of Cyclospora in Produce

ONLINE & OF NOTE
FDA has published a new webpage listing retail food safety resources and information. The webpage categorizes FDA's most popular retail food safety information in a table format sortable by topic, title, type of document, and languages available. Resources listed on the webpage include scientific articles, databases, factsheets, posters, guides, handbooks, and other tools.
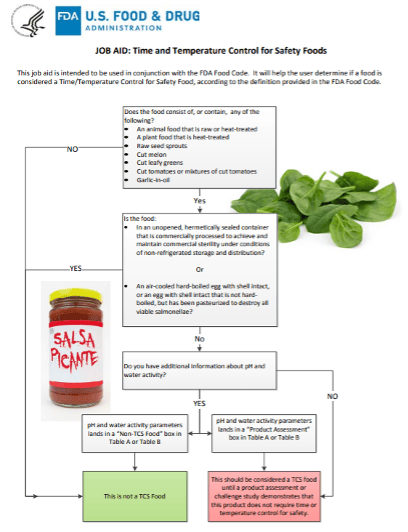
With the release of the new webpage, FDA also highlighted a new resource, titled, "FDA's Job Aid: Time and Temperature Control for Safety Foods" (pictured). The job aid is intended to be used in conjunction with the FDA Food Code to help the user determine if a food is considered a Time/Temperature Control for Safety Food, according to the definition provided in the Food Code.
Listing of Retail Food Protection Information and Resources: www.fda.gov/food/retail-food-protection/listing-retail-food-protection-information-and-resources
Job Aid: Time and Temperature Control for Safety Foods: https://www.fda.gov/media/101004/download
FDA Releases New Webpage for Retail Food Safety Resources, Food Code Job Aid