SCROLL DOWN
A Critical Look at Reducing the Risk of Salmonella in Poultry Products
There is a need to address Salmonella at pre-harvest, in addition to the current practice of minimizing the risk during processing
By Harshavardhan Thippareddi, Ph.D., and Manpreet Singh, Ph.D.
Video credit: SVTeam/Creatas Video+ / Getty Images Plus via Getty Images
> SPOTLIGHT
SCROLL DOWN
Survey of Preventive Measures for Controlling Foodborne Parasites
A globalized food supply system brings the “invisible risk” into focus
By Larry Keener, CFS, PA, PCQI, and Tatiana Koutchma, Ph.D.
> FOODBORNE PARASITES
Video credit: tunart/Creatas Video via Getty Images
SCROLL DOWN
Poultry meat consumption has significantly increased over the past three decades in the U.S., and it is currently the most consumed meat protein compared to beef and pork. This increase has occurred gradually due to significant scientific research and advances in genetics, nutrition, and bird management and husbandry, resulting in reduced processing costs and, eventually, decreased meat costs for the consumer. This increase in consumption has come at a cost to the consumer, as well—the risk of salmonellosis related to higher consumption. The U.S. Centers for Disease Control and Prevention has reported that the incidence of salmonellosis has not declined, despite a reduction in Salmonella prevalence in poultry and poultry products. This may be related to several other foods being sources of Salmonella—for example, produce.
Poultry meat has been associated with higher risk of salmonellosis compared to other meat species (such as beef and pork), resulting from the ability of the microorganism to colonize the gastrointestinal (GI) tract of the birds, which leads to higher prevalence of Salmonella in the poultry meat after processing. Current food safety regulations impose performance standards for Salmonella on poultry meat subsequent to processing, based on the prevalence of Salmonella on both the whole bird and poultry parts. Processors have incorporated several antimicrobial intervention steps in poultry meat processing to reduce the risk of Salmonella.
While Salmonella prevalence has decreased significantly over the past three decades due to these processing changes, human illness cases from Salmonella have not decreased proportionally. To understand and eventually reduce the risk of salmonellosis from poultry meat, it is necessary to understand poultry production systems; the introduction of the microorganism into the poultry ecosystem, as well as the bird GI tract; the sources of Salmonella during production; and strategies to control or reduce the risk from this microorganism, both at the pre- and post-harvest stages. In a series of three articles, we will take a critical look at the sources of the microorganism, pre-harvest control at the broiler grow-out farm, and post-harvest control of Salmonella during poultry processing.
The mandate for the implementation of Hazard Analysis and Critical Control Points (HACCP) systems in meat and poultry processing operations by the U.S. Department of Agriculture (USDA) Food Safety and Inspection Service (FSIS) in 1999 has resulted in the transformation of the industry in terms of addressing food safety hazards, specifically biological hazards such as Salmonella and Campylobacter in poultry. This mandate required the industry to reduce the risk of food safety hazards through identification of critical control points (CCPs) within the processing system and control the hazards at those specific steps to meet regulatory performance standards for Salmonella. These performance standards were enforced subsequent to poultry processing, after the chilling step, where the broiler carcasses were collected by USDA's FSIS to determine Salmonella prevalence.
In response, several poultry processing operations have improved their control of the antimicrobial intervention step (which is most often a CCP) and incorporated several other antimicrobial interventions, where the reduction in Salmonella concentrations and/or prevalence was achieved. USDA's FSIS established performance standards for Salmonella prevalence subsequent to processing (chilling), requiring processors to meet this regulatory standard. For example, the Salmonella performance standard for broilers was set at 20 percent prevalence, indicating that in the samples (whole bird rinse) collected, no more than 20 percent of the samples can be positive for Salmonella. Within a few years of the HACCP mandate, the majority of the large poultry processors were in compliance with the Salmonella performance standard, with several achieving a greater level of compliance (i.e., < 50 percent Salmonella prevalence [< 10 percent Salmonella prevalence] compared to the performance standard).
More recently, USDA's FSIS has revised the performance standard for Salmonella in poultry, along with the incorporation of Campylobacter standards and performance standards for Salmonella and Campylobacter in chicken parts. While the majority of the poultry processors do meet the performance standards, to further reduce the prevalence and/or concentrations of the microorganism, there is a need to address it at the pre-harvest stage. Further reductions can be achieved only by reducing the prevalence and concentrations of the microorganisms in the broilers presented for slaughter.
Poultry and Salmonella
The genus Salmonella consists of two species: enterica and bongori. The majority of the serovars that are of zoonotic origin can be divided based on host range into host-adapted and ubiquitous (non-adapted). The host-adapted serovar for poultry, Gallinarum, has been controlled in poultry flocks through surveillance and vaccination programs in the U.S. The majority of the foodborne serovars of Salmonella include the more than 2,400 ubiquitous serotypes, although only a few are isolated frequently from commercial poultry and poultry products.
Salmonella enterica serotypes can cause systemic disease, gastroenteritis and disease-free gastric colonization. More often, the serotypes isolated from poultry represent those that colonize the gastrointestinal tract with minimal host-related pathogenesis and potentially cause human illness. The current regulatory environment in the U.S. considers all Salmonella as potential human pathogens, and no serovar distinctions are made in terms of prevalence in poultry and related performance standards for whole carcasses (chicken/broiler), as well as for chicken parts.
Sources of Salmonella in Poultry
Salmonella can enter the poultry gut ecosystem through a variety of sources including feed, water, litter (fresh and used), pests (including darkling beetles), rodents, surrounding environment (animals and wild birds), and colonize the GI tract of the birds. Once colonized, elimination of Salmonella from the bird is almost impossible. To achieve better control of Salmonella in the poultry meat, it is necessary to understand the sources of the microorganism in the poultry production system and the contributions of these sources (Figure 1).
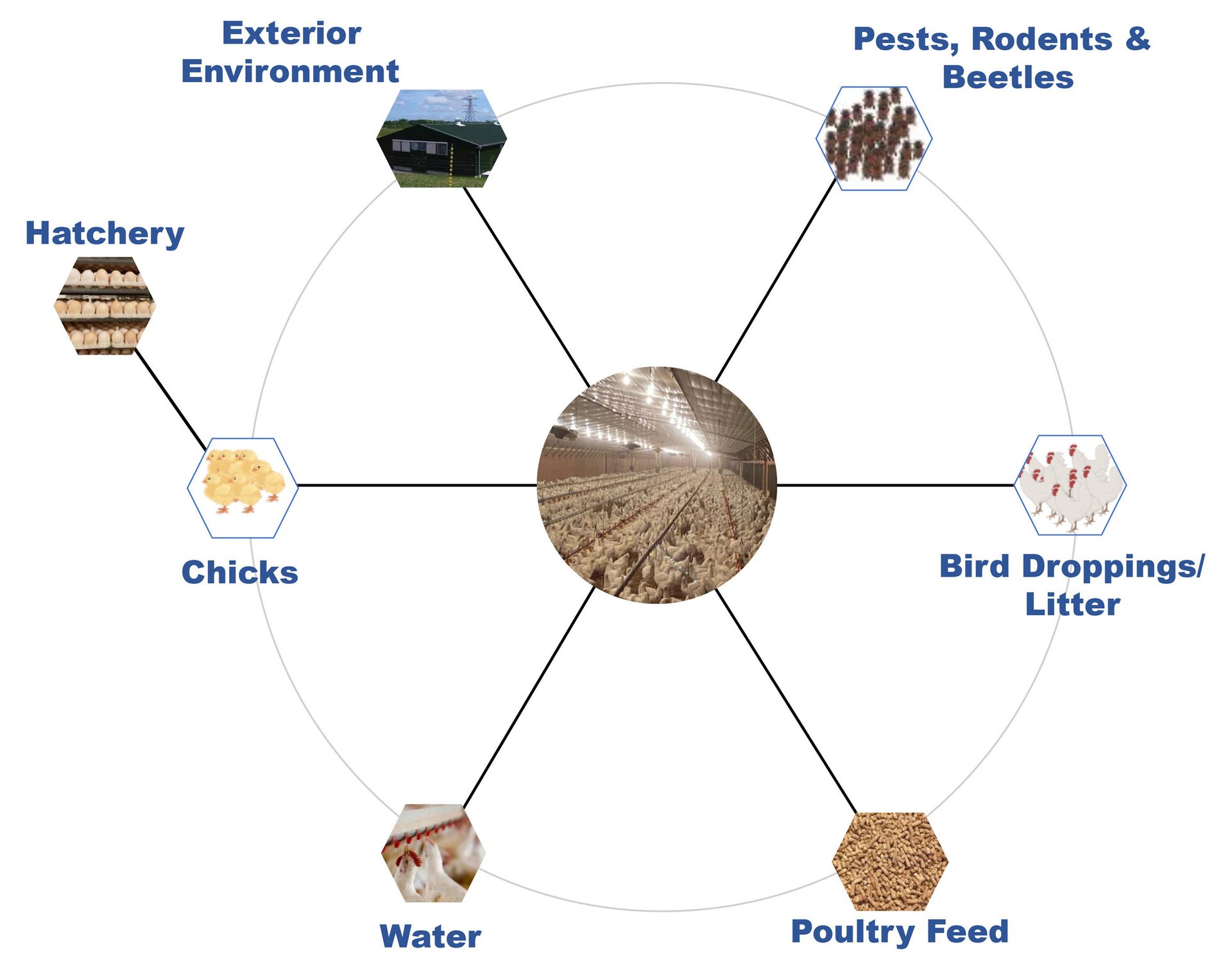
FIGURE 1. Sources of Salmonella in the Poultry Production System
Poultry House External Environment
The surroundings of the poultry house can be a major source of Salmonella as wild birds, domestic animals (including meat animals), as well as pets can be sources of the microorganism and can gain entry through personnel entering the poultry house for bird management and upkeep. If properly implemented, biosecurity measures can play a significant role in minimizing the risk of Salmonella entry into the poultry house. Measures critical to addressing this source of Salmonella include personnel/visitor control, minimizing contact with domestic and wild animals, upkeep of the immediate surroundings of the poultry house to prevent rodent infestation, and minimizing movement of equipment and transport vehicles between farms and personnel.
Anecdotally, implementing and adhering to strict biosecurity principles has been observed to reduce the prevalence of other poultry diseases in commercial poultry, and the same principles can be effective against carryover of Salmonella into the poultry house and subsequent colonization of the bird GI tract.
Poultry Feed
Poultry feed has been implicated in the cross-continental spread of some Salmonella serovars, such as Agona, through fish meal produced in South America and imported into Europe. Poultry feed ingredients contain sources of energy (carbohydrates from cereal grains including corn, wheat, rice, sorghum, barley, etc.), protein (vegetable sources such as soybean meal, canola meal, cottonseed meal, sunflower meal, etc.; and animal sources such as meat meal, meat and bone meal, poultry byproduct meal, blood meal, feather meal, etc.), fats and oils (tallow, soy, palm, poultry fat, etc.), minerals, and vitamins. Of these, the animal protein components have been historically associated with higher prevalence of Salmonella compared to the other protein (soy meal) and fat (soy or corn oil, rendered poultry fat, tallow, etc.) sources, although all of them can be sources of foodborne pathogens such as Salmonella. While the risk of Salmonella prevalence is low in cereal grains and their products, these, along with the flour prepared from cereals, have been implicated in salmonellosis outbreaks and involved in several flour recalls, indicating the presence of the pathogen on or in the cereals or contamination during milling and/or packaging.
The conventional rendering process for the animal protein meal manufacturing should be adequate to destroy all vegetative forms of foodborne pathogens, including Salmonella. As such, contamination of animal protein with vegetative foodborne pathogens, such as Salmonella, indicates recontamination of the prepared product from the environment from a variety of sources, including air, equipment, packaging material, etc. Although the feed ingredients may carry pathogens, the feed manufacturing process itself may be adequate to reduce the populations of Salmonella and other vegetative pathogens based on the type of feed manufactured—mash versus pellet. The pelleting temperatures may be adequate in some cases to reduce and/or eliminate Salmonella in the finished poultry feed.
Similar to rendering, even if the pelleting process is adequate to reduce Salmonella populations brought in by the feed ingredients, recontamination can occur from the feed processing environment including the air, feed handling and storage equipment, etc. If the feed manufacturing process is adequate to reduce the Salmonella populations, then reasonable efforts should be made to reduce the risk of cross-contamination or recontamination of the manufactured feed.
Hatchery
Egg shell surfaces can be contaminated with Salmonella, as the eggs must pass from the oviduct and eventually the cloaca. Immediately after the egg is laid, the egg cools from the body temperature of the hen to ambient temperature, causing the egg contents to shrink and, thus, draw the surface-deposited microorganisms, including Salmonella (if present), to the interior of the shell and potentially the egg shell membranes. As Salmonella can survive for extended periods of time under low-moisture, dry environmental conditions, the microorganism can persist through hatching and be a source for the chicks to peck immediately after hatching.
Hatcheries today have highly automated systems with high output of chicks, and can contribute to the spread of Salmonella to the chicks. Salmonella has been isolated from several surfaces in hatcheries, such as hatchers, chick handling areas, cleaning equipment, setters, egg transfer areas, and service areas, among others. Large amounts of airborne fluff and dust generated during the hatching process were shown to be the main sources of Salmonella in the chicks and, eventually, the broilers. Some studies have shown that Salmonella serovars that are predominant in the hatchery also predominate at the processing facilities, indicating a potential link.
Chicks
Salmonella can be transmitted vertically as well as horizontally in poultry, indicating that Salmonella can be transferred through the egg to the progeny (chicks), as well as from among birds during grow-out. While vertical transmission through the egg is possible, the majority of the Salmonella infection and propagation probably occurs through eggshell contamination and aerosolization during hatching. The chicks can be infected by ingestion of Salmonella-contaminated membranes and other egg material, or through the respiratory system from aerosol or from contaminated fluff and other material in the hatcher. Day-old chicks are more susceptible to Salmonella colonization compared to three-day-old chicks. While sampling of chicks and their GI tracts may not reveal the extent of colonization, sampling of the same chicks at later dates reveals greater prevalence, indicating the probability of low concentrations of Salmonella in the early stages of exposure of Salmonella-contaminated material and environment in the hatcheries.
"Controlling the entry of Salmonella into the poultry house and colonization of the bird gut is critical for minimizing the risk of Salmonella from poultry."
Poultry House Interior Environment
Poultry house interiors can be significant sources of Salmonella through vectors such as insects (darkling beetles and flies), rodents, and others that have established in the poultry house. The birds are exposed to Salmonella, resulting in gut colonization. Once the bird gut is colonized, the bird sheds Salmonella in its fecal droppings, serving as a further source of Salmonella spread within the flock.
- Darkling beetles: Darkling beetles and their larvae, lesser meal worms that have established in the poultry houses, have been shown to be significant vectors for Salmonella for broilers during grow-out. Studies have shown that Salmonella can survive in the beetles for up to two weeks and throughout the lifecycle of the darkling beetle.
- Rodents (mice): Rodents can be a persistent issue for poultry farmers and are probably the most versatile among the pests. The mouse gut can be colonized by Salmonella and shed in the droppings, along with the birds on the same litter. Birds, being coprophagic, ingest these Salmonella-contaminated droppings and can be colonized over time. Salmonella prevalence rates have been reported up to 40 percent in roof rats in poultry houses. Mice can persist in the poultry house environment for years, even with good pest control measures.
- Other interior environment: Interior poultry house environment surfaces, such as dust settling on surfaces, fan blades, interior surfaces, and walls, can contain Salmonella and may be a source of the microorganism for the birds and other vectors.
Water
Water supply in a poultry farm can come from municipal or treated well water, with the recommendation to be of potable quality. The risk of contamination of the water from the source can be minimal, although water contamination with foodborne pathogens has been reported. Low environmental temperature, low flow rates of water in the pipeline, and adequate nutrients make the water supply lines in the poultry house ideal for microbial growth and establishment of biofilms inside the pipelines. However, the source of Salmonella in the water can be from the dust in the house environment or the birds themselves. While the poultry farmers clean and sanitize the drinking waterlines, these activities may not be performed frequently enough to eliminate the water as a source of the microorganism in the poultry house environment.
Bird Droppings and Litter
Broiler litter is a mixture of organic material, such as wood shavings, peanut hulls, rice straw, or husks, along with feces from the previous flocks. Farmers often reuse the litter for multiple flocks, as the cost of litter can be high, and the time and effort required to replace the litter can be intensive. The litter can be contaminated with Salmonella from darkling beetles, rodent droppings, or the few chicks that may have been infected with the microorganism at the hatchery.
Once the birds are colonized with the microorganism, within days, they start shedding Salmonella in large numbers in their droppings. Although the droppings may dry over time, the ability of Salmonella to survive for long periods of time in dry environments constitutes a major risk for colonization of other birds within the same flock. Salmonella can survive in the litter between flocks and be a source for colonization of the chicks and, subsequently, the birds of the following flock.
Summary and Conclusions
The poultry house environment is replete with sources of Salmonella. Colonization of the bird gut is mostly through the fecal-oral route. Controlling the entry of Salmonella into the poultry house and colonization of the bird gut is critical for minimizing the risk of Salmonella from poultry. Exposure to Salmonella from the numerous vectors for Salmonella in the poultry production system, and the coprophagic nature of chickens, result in the ingestion of fecal droppings from colonized birds to several non-colonized birds. Once Salmonella colonization of the bird gut occurs in a few birds, exponential spread to other birds within the flock ensues within a short period of time, increasing the prevalence.
While the concentrations of the Salmonella cells shed in the fecal droppings may decrease from the time of first colonization, Salmonella can remain in the gut of the bird, posing a risk of cross-contamination during harvest at the processing plant. Reducing the risk of Salmonella requires reduction in the concentrations of cells in the bird gut and on the surface of the bird presented at slaughter. There is a need to address Salmonella at pre-harvest (i.e., at the farm level), in addition to the current practice of minimizing the risk during processing.
Harshavardhan Thippareddi, Ph.D., is the John Bekkers Professor of Poultry Science in the Department of Poultry Science at the University of Georgia in Athens, Georgia.
Manpreet Singh, Ph.D., is Professor and Head of the Department of Food Science and Technology at the University of Georgia in Athens, Georgia.

