SCROLL
DOWN
The U.S. Food and Drug Administration (FDA) has provided updates on its proposal to create a unified Human Foods Program (HFP), which includes a new model for the Office of Regulatory Affairs (ORA). These decisions are intended to enhance coordination, prevention, and response activities across the FDA, and were identified by a cross-cutting working group of agency officials with expertise in different functional and operational areas.
The agency has provided high-level organization charts to reflect the changes that are being proposed as part of the unified HFP and new ORA model. To enhance clarity around the proposed core mission of ORA, the FDA is also considering a renaming effort for this office to more appropriately align its title to the structure and functional duties of the agency's field operations.
FDA is proposing a number of changes to ORA, including moving several of the office's laboratories and merging its current compliance functions into those of the new HFP and other agency product centers. Based on recommendations from the working group and from the external evaluation conducted by the Reagan-Udall Foundation, FDA is proposing the following additional changes:
- Establishing ORA's core mission as conducting investigations, inspections, and imports for all FDA-regulated products, with assignments planned in partnership with the HFP and other product programs or centers. The new Deputy Commissioner for Human Foods will have oversight of all budget and resource allocations for the entire HFP, including ORA resources.
- Merging compliance functions currently managed within ORA into the HFP and the product centers' existing compliance functions to streamline operations and expedite decision-making.
- Realigning the eight Human and Animal Food laboratories that are currently managed by ORA into the HFP. These eight labs will team up with the four labs in FDA's current Center for Food Safety and Applied Nutrition (CFSAN) to form a unified food laboratory enterprise under the HFP. The labs will report to a member of the executive leadership team under the Deputy Commissioner for Human Foods, who will work closely with the Chief Scientist and the Center for Veterinary Medicine (CVM) director to coordinate on research priorities.
- Transitioning certain functions under the Office of Security and Emergency Management, currently in the Office of Operations, to ORA. This includes the Office of Emergency Management, which activates Incident Management Groups with augmented staffing from relevant Centers and Offices to monitor and manage coordinated responses to emergency situations, such as emergencies involving regulated products like recalls, hurricanes, fires, floods, and other incidents.
- As previously shared, unifying state and local food safety partnership functions and certain aspects of international food safety partnerships into an Office of Integrated Food Safety System Partnerships in the HFP. This office will report to a member of the executive leadership team under the Deputy Commissioner for Human Foods, who will closely collaborate with the CVM director to advance a truly integrated food safety system.
- Reviewing support functions across ORA and proposing realignment of certain resources and personnel to support these changes. This includes staff and resources in ORA's Office of Regulatory Management Operations, Office of Information Systems Management, Office of Training, and Office of Communications and Project Management.
- Prioritizing recruitment, retention and training opportunities for field-based employees with the availability of Title 21 hiring authority to support the agency's ongoing efforts to increase its inspectional activities domestically and internationally.
These proposed changes align with many of the recommendations from the Reagan-Udall Foundation evaluation, as well as a separate internal review of the agency's infant formula response completed last year. They also empower the Deputy Commissioner for Human Foods to have full authority over, and set the strategic direction of, all foods-related resources.
FDA is in the final stages of the recruitment process for the Deputy Commissioner for Human Foods and will be providing an update in the near future. FDA remains on target to finalize its reorganization proposal, for both ORA and the unified HFP, this autumn.

Latest Update on FDA Human Foods Reorganization Reveals Major Changes to ORA
Cronobacter to be Added to List of Nationally Notifiable Diseases
Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images

On June 29, 2023, the Council of State and Territorial Epidemiologists (CTSE) voted to make Cronobacter sakazakii a nationally notifiable disease, requiring health departments in the U.S. to track and report cases of C. sakazakii to the U.S. Centers for Disease Control and Prevention (CDC). The decision follows a prolific foodborne illness outbreak linked to powdered infant formula products contaminated by the pathogen that occurred in 2022, and a subsequent large-scale recall that caused a national shortage of the crucial food.
C. sakazakii is set to be added to the list of nationally notifiable diseases in 2024, after which point doctors and laboratories will be required to report cases of infection in infants less than one year of age to state health departments. The decision was advocated for by many stakeholder groups, as well as by the U.S. Food and Drug Administration in its November 2022 prevention strategy for C. sakazakii in powdered infant formula. Other foodborne pathogens on the list of nationally notifiable diseases include Salmonella, Escherichia coli, and Listeria monocytogenes.
Based on an assessment of the impact of glyphosate on the health of humans, animals, and the environment, the European Food Safety Authority (EFSA) has determined that there do not exist any critical areas of concern. However, some data gaps were identified in EFSA's conclusions for the European Commission and EU Member States to consider in the next stage of the renewal of approval process for the chemical.
A concern is defined as critical when it affects all proposed uses of the active substance under evaluation (e.g., pre-sowing uses, post-harvest uses, and etc.), thus preventing its approval or renewal.
Glyphosate is a chemical substance used in a number of herbicide products, and its use in Europe is subject to strict regulation. Glyphosate is currently approved for use in the EU until December 15, 2023. The risk assessment by Member States and the subsequent peer review by EFSA was carried out as part of the legal process to renew the approval of its use in Europe.
In 2022, the European Chemicals Agency (ECHA) carried out a hazard assessment of glyphosate and concluded that it did not meet the scientific criteria to be classified as a carcinogenic, mutagenic, or reprotoxic substance. EFSA used ECHA's hazard classification for the purposes of the EU risk assessment on glyphosate. Where data gaps are identified, they are reported in EFSA's conclusions as either issues that could not be finalized or outstanding issues.
Issues that could not be finalized include the assessment of one of the impurities in glyphosate, the consumer dietary risk assessment, and the assessment of risks to aquatic plants. Outstanding issues include, but are not limited to, a lack of information about the toxicity of one of the components present in the glyphosate-based pesticide formulation submitted for evaluation, which is needed to conclude the risk assessment of the formulation for representative uses. For the formulation, there were no indications of acute toxicity and genotoxicity.
Regarding biodiversity, experts recognized that the risks associated with the representative uses of glyphosate are complex and depend on multiple factors. They also noted a lack of harmonized methodologies and agreed specific protection goals. Overall, the available information does not allow firm conclusions to be drawn on this aspect of the risk assessment, and risk managers are encouraged to consider mitigation measures. Additionally, with respect to ecotoxicology, the data package allowed a conservative risk assessment approach, which identified a high long-term risk to mammals in 12 out of 23 proposed uses of glyphosate.
EFSA's conclusions on the peer review of the risk assessment for glyphosate have been shared with the European Commission and Member States to inform the decision they will take about whether to keep glyphosate on the EU list of approved pesticide active substances.

EFSA Finds No Critical Health Concerns for Herbicide Glyphosate
FDA Releases New Food Fraud Webpage
The U.S. Food and Drug Administration (FDA) has released a new website on economically motivated adulteration (EMA), including food fraud. The purpose of the website is to keep businesses and consumers informed on the latest food fraud developments.
The website includes links on how to report food fraud; examples of food adulteration; how food fraud is detected and monitored; enforcement and legal consequences, such as recalls, seizures, and import refusals; guidance documents to assist manufacturers and importers; and a list of import alerts.
EMA occurs when "someone intentionally leaves out, takes out, or substitutes a valuable ingredient or part of a food," according to FDA. EMA also occurs when a substance is added to a food to make it appear better or of greater value.
Food fraud is a common type of EMA that FDA investigates, but EMA also occurs with other products, including animal food and cosmetics. Some types of EMA are also misbranding violations. Estimating how frequently food fraud occurs or its exact economic impact can be challenging because food fraud is designed to avoid detection. Outside estimates by experts have found that food fraud affects 1 percent of the global food industry at a cost of approximately $10–$15 billion per year, although more recent expert estimates peg the cost as high as $40 billion per year.
Food fraud can also lead to major health issues and even death. Some examples include lead poisoning from adulterated spices and allergic reactions to a hidden or substituted ingredient that contains a small amount of just one food allergen.
Click here to visit FDA’s new EMA website.

Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images
Approximately 40 percent of foodborne illness outbreaks in the U.S. associated with retail food establishments during 2017–2019 were caused by an infectious employee, according to the U.S. Centers for Disease Control and Prevention (CDC).
An entry in CDC's Morbidity and Mortality Weekly Report summarized environmental health data that was collected during retail foodborne outbreak investigations and was submitted to the National Environmental Assessment Reporting System (NEARS). NEARS was launched in 2014 to compliment National Outbreak Reporting System (NORS) surveillance. State and local health departments voluntarily enter data from their foodborne illness outbreak investigations of retail food establishments into NEARS.
During 2017–2019, a total of 800 foodborne illness outbreaks linked to 875 retail food establishments were reported to NEARS by 25 state and local health departments. Of the 800 outbreaks, 555 had a confirmed or suspected agent. Norovirus and Salmonella accounted for 47 and 18.6 percent of the outbreaks, respectively.
CDC also found that approximately 40 percent of outbreaks with identified contributing factors had at least one reported factor associated with food contamination by an ill or infectious food worker. In interviews, the vast majority (91.7 percent) of retail food managers reported that their establishment had a policy requiring food workers to notify their manager when they were ill. Additionally, 66 percent of the 725 managers interviewed stated that their sick employee policy was written, and only 23 percent said their policy included a complete list of illness symptoms about which workers were required to notify managers. Although 85.5 percent of interviewees said that their establishment had a policy restricting or excluding ill workers from working, only 16.1 percent of establishments linked to outbreaks had policies addressing all four components relating to ill or infectious workers, which are:
- Policy requires workers to notify a manager when they are ill
- Policy specifies all five illness symptoms about which workers need to notify managers (vomiting, diarrhea, jaundice, sore throat with fever, and lesion with pus)
- Policy restricts or excludes ill workers from working
- Policy specifies all five illness symptoms requiring restriction or exclusion from work.
Based on its report, CDC suggests that the content and enforcement of existing sick policies might need to be reexamined and refined. Future analyses of data may help guide the development of effective contamination prevention strategies by describing how establishments' characteristics and food safety policies and practices relate to foodborne illness outbreaks.

40 Percent of Retail Foodborne Illness Outbreaks Linked to Sick Employees, Says CDC
Following a One Health approach, Canadian federal, provincial, and territorial (FPT) governments have made a five-year commitment for concerted action to tackle antimicrobial resistance (AMR) through ten shared priority actions across five pillars. The action plan was developed by the FPT Steering Committee, which consisted of government representatives from the public health, human and animal health, agriculture, and agrifood sectors.
Nearly 15 people in Canada per day were estimated to have lost their lives to resistant infections in 2018, and, in the same year, the economic burden of AMR on Canada's healthcare system and gross domestic product (GDP) totaled $1.4 billion and $2 billion, respectively. There is global consensus that AMR must be addressed by adopting a One Health approach, meaning that actions to limit the emergence and spread of AMR must be multi-sectoral and consider the close interplay between humans, animals, crops, and the shared environment.
The overuse of antibiotics, including medically important antimicrobials (MIA), in animal agriculture for growth promotion and other purposes are major contributors to the global rise of AMR. In 2020, 82 percent of all MIAs sold by volume in Canada were intended for use in food-producing animals and horses—approximately 1.8 times more than was used for treating humans (a weighted estimate, taking the large number of animals compared to humans in Canada into account). However, surveillance of antimicrobial use (AMU) on broiler chicken, grower-finisher pig, and turkey farms have indicated a decrease in AMU from 2016–2020.
The action plan provides a five-year (2023–2027) blueprint for strengthening Canada's collective AMR preparedness and response across the One Health spectrum. The action plan reflects the commitment of health and agriculture leaders in governments across Canada and responds to calls to action from non-governmental organizations, the healthcare and veterinary sectors, the scientific and research community, and industry to work together to combat the growing threat of AMR. The action plan outlines ten priority actions that will guide FPT governments' AMR efforts across five pillars:
- Research and innovation
- Achieve improved, sustainable access to antimicrobials, diagnostics, and alternatives to antimicrobials to better mitigate AMR
- Expand scientific knowledge base and tools to inform effective AMR/AMU interventions
- Surveillance
- Cultivate robust, integrated One Health AMR and AMU surveillance infrastructure with data that are accessible, reliable, timely, nationally representative, and capable of detecting emerging threats
- Comprehensively understand AMR and AMU trends at national, regional, and local levels to support evidence-based decision-making and to monitor the impacts of interventions
- Stewardship
- Give prescribers and other professionals in Canada the resources, training, and tools to facilitate appropriate AMU in humans and animals
- Foster understanding among Canadians of the importance of the appropriate use of antimicrobials
- Infection Prevention and Control (IPC)
- Increase effective implementation of infection prevention across community and institutional health sectors, including for populations disproportionately impacted by AMR
- Improve animal health and food safety along the farm-to-fork continuum to prevent and limit the spread of infection and foodborne pathogens
- Leadership
- Build on existing One Health AMR governance structures to create a "network of networks" with inclusive representation to support action plan implementation
- Increase Canada's contributions to global efforts to advance key bilateral and multilateral commitments by prioritizing: generating improved data/evidence on AMR and AMU and strengthening surveillance systems and data standards, and expanding efforts to support low- and middle-income countries by advancing equitable access, stewardship, and IPC initiatives.
The action plan is designed to encourage jurisdictions, sectors, and disciplines across Canada to easily identify how and where they can best contribute to collective efforts to address AMR and inappropriate AMU within their unique contexts and for their specific stakeholders. The agriculture and agrifood industries are tasked with advancing appropriate AMU, promoting IPC and good animal management practices, and establishing some control programs in farmed animal production to protect the health of animals and to preserve the quality of the food supply.

Canada Makes Five-Year Commitment to Tackling AMR Through One Health Approach

The International Fresh Produce Association (IFPA) has announced Natalie Dyenson as its new Chief Regulatory and Food Safety Officer.
The Consumer Goods Forum (CGF), parent organization of the Global Food Safety Initiative (GFSI), has announced Frans Muller, President and CEO of Ahold Delhaize, and Dirk Van de Put, Chairman and CEO of Mondelēz International, as CGF's new Co-Chairs for the next two years.
The Board of Directors for FoodChain ID has appointed Conor Kearney as CEO. Mr. Kearney succeeds Brad Riemenapp, who led FoodChain ID as CEO for more than five years before passing away in May.
BoMill AB has announced the appointment of Hansi Biedermann to the newly established role of Business Development Manager.
Land O'Frost Inc., has named Dindy Williams as the Plant Manager of the company's Searcy, Arkansas facility. Williams, who started with the company in 1982, is the first woman to hold this position at Land O'Frost.
Nuritas has hired John Casey as its new Chief Manufacturing and Supply Chain Officer.
ProEgg, a farmer-owned cooperative of experienced egg producers with farms across the U.S., has named Jerry Wilkins as Vice President of Sales.
PPG Packaging Coatings has appointed Ethan Schneider as the Global Beverage Segment Manager of its Packaging Coatings business.
Clever Carnivore has hired Tyson veteran Russell Thomas as its Vice President of Product Development.
IMA Dairy & Food USA has hired automation specialist Alex Donovan as its West Coast Sales Manager.
The Western Association of Food and Drug Officials (WAFDO) changed its bylaws to appoint Seattle Fish Company's Ken Boyer as the Association's 2023 President. Boyer is the first industry member to hold an officer position at WAFDO.
BIOIONIX LLC has appointed Ryan Karns as its new CEO.
PSSI has appointed Vipul Soni as its new CFO.
Detectamet has announced Steve Herd as its CCO.

DYENSON

KEARNEY

BIEDERMANN

WILLIAMS

SCHNEIDER
Rheonix Develops Fully Automated Cyclospora Test in Collaboration with FDA
Rheonix Inc., a manufacturer of microfluidic workstations and assays for molecular testing, announced that it has completed development of a fully automated test for the detection of Cyclospora cayetanensis in food and environmental samples. The development was conducted in collaboration with scientists at the Office of Applied Research and Safety Assessment (OARSA) within the Center for Food Safety and Applied Nutrition (CFSAN) of the Food and Drug Administration (FDA).
The assay is based on genetic targets published by FDA, and is processed using Rheonix reagents and consumables on the Rheonix Encompass Optimum™ workstation. The fully automated method enables detection of low levels of Cyclospora cayetanensis, saving approximately four hours of hands-on time per 24-sample run compared with the existing workflow. The OARSA team, with assistance from the Rheonix team, will validate the assay on a range of food and environmental sample types.

The International Association for Food Protection (IAFP) announced the Compass Group North America as the 2023 recipient of its Black Pearl Award, presented at IAFP's Annual Meeting in Toronto, Ontario, Canada, July 16–19. The Black Pearl Award is given annually to one company for its efforts in advancing food safety and quality through consumer programs, employee relations, educational activities, adherence to standards, and support of the goals and objectives of IAFP. Compass Group is a contract food service business that operates in tens of thousands of food service locations and employs hundreds of thousands of associates in the U.S. alone. The company's comprehensive food safety system covers four pillars: Food Safety, Food Quality, Food Fraud, and Food Defense.
IAFP Honors Compass Group North America with 2023 Black Pearl Award
The Association of Food and Drug Officials (AFDO) has announced its new Food Safety Regulatory Professional Credential Series exclusively to local, state, tribal, territorial, and federal regulators. Through the program, food safety regulatory professionals can be recognized for their mastery of core competencies and skills based upon the U.S. Food and Drug Administration's National Curriculum Standards (FDA's NCS). The tiered program begins with Food Safety Regulator Certification and Food Foundations Regulator Certification. The series also includes specialized advanced certifications for both manufactured and retail foods. The first two certifications are required before completing the advanced offerings. Professional Credential Series can be earned any day, at any time, assessed either in-person or through Live+ proctored testing.

AFDO Offers Food Safety Regulatory Professional Credential Series Based on FDA Curriculum Standards
GS1 US has published a new guideline to assist the U.S. foodservice industry in using radio frequency identification (RFID) to improve supply chain visibility, efficiency, and consumer safety. The "GS1 US RFID Foodservice Implementation Guideline" provides case/carton requirements for foodservice suppliers to minimize disparate supplier tagging requirements. For food products and consumer-facing food packaging, the guideline specifies tag encoding, tag marking, and tag placement.
The new guideline was developed by a GS1 US foodservice workgroup to identify how suppliers should encode GS1 Standards in RAIN RFID tags using GS1's Electronic Product Code (EPC) schemes outlined in the EPC Tag Data Standard (TDS) for automatic data capture, and to provide a roadmap for adoption. The new document is designed to guide companies with implementing open, interoperable GS1 Standards to enable more efficient tracking, management, and traceability of products throughout the supply chain. Distributors can learn how to integrate RFID technology within their systems to ensure compliance with new standards, and end users can benefit from understanding the available data and the access this enhanced method of data capture provides.

GS1 US Issues Guidance for Foodservice Use of RFID for Product Traceability
The National Institute of Oilseed Products (NIOP) has formed a Product Integrity Committee (PIC) in response to increasing incidents of food fraud within the oilseed industry worldwide. The committee will closely investigate the issue of food fraud, collect data from member companies, and educate local, state, and federal leaders responsible for overseeing the health, safety, and labeling of packaged goods on why the critical issue must be addressed. NIOP members have also begun quality testing oil shipments at many of its members' facilities, which will provide additional data on the prevalence of fraud within the industry.
Oilseed fraud typically occurs by short weighting products or substituting high-quality oils with inferior oils to increase profit margins. Fraudulent oils may have an inferior nutritional quality and value for consumers. In establishing the PIC, NIOP has elected to become a comprehensive resource offering the latest news and information on food fraud within the oilseed industry, and to help its members advocate for meaningful policy enforcement and updates for protecting the integrity of weight and ingredients in packaged products.
NIOP Establishes Product Integrity Committee to Address Food Fraud


ONLINE & OF NOTE
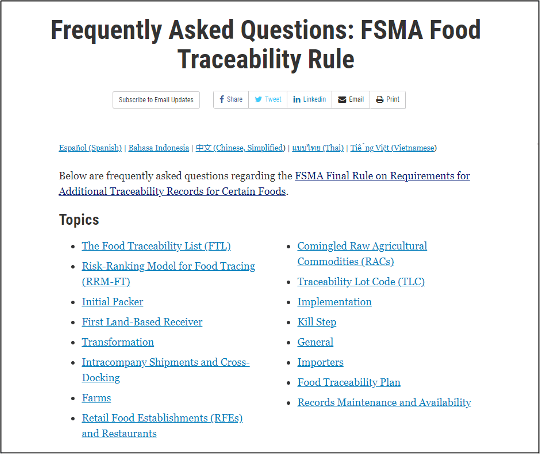
The U.S. Food and Drug Administration (FDA) has posted answers to frequently asked questions (FAQs) and additional tools to provide industry with more information about the FDA Food Safety Modernization Act (FSMA) Food Traceability Final Rule. The new FAQs address questions to help clarify how the rule applies to specific situations and are largely based on questions received during FDA's regular interactions with industry and on questions sent via the Technical Assistance Network, a central source of information for questions related to FSMA rules, programs, and implementation strategies.
Along with FAQs, additional tools developed to help educate and inform industry subject to the Food Traceability Final Rule are accessible from FDA’s traceability website, including:
- The results for all foods and associated commodity-hazard pairs included in the Risk-Ranking Model for Food Tracing, beyond what appear on the food traceability list (FTL)
- Additional description on the FTL webpage to clarify that "nut butters" include all forms of nut butters: shelf-stable, refrigerated, and frozen products
- Eight new supply chain examples that illustrate how the rule is applied in different scenarios for different commodities
- Fact sheets: Recordkeeping Information for Produce Farms and Coverage and Exemption for Produce Farms
- A "Guide to Getting Started with the Food Traceability Rule"
- Additional foreign language translations of the Critical Tracking Event and Key Data Elements interactive tool and supply chain examples.
FDA has also published a Small Entity Compliance Guide for FSMA 204. The compliance date for all those subject to the Food Traceability Rule is January 20, 2026.