Testing and Analysis
By Mohit Verma, Ph.D., Assistant Professor, Department of Agricultural and Biological Engineering and Weldon School of Biomedical Engineering, Purdue University
Quantifying the Invisible: New Tools to Evaluate Bioaerosol Risks in Fresh Produce
Recent advances in biosensing and molecular diagnostics are allowing producers to better quantify the threat from bioaerosols at the field level

Image credit: MJPS/ iStock/Getty Images Plus via Getty Images
SCROLL DOWN
The safety of fresh produce hinges on a complex web of environmental and operational factors. Among these, proximity to concentrated animal feeding operations (CAFOs) is a persistent challenge. While existing guidance from the California and Arizona Leafy Greens Marketing Agreements (LGMAs)1,2 offers critical recommendations—particularly on buffer distances and standard operating procedures (SOPs)—distance alone is not always sufficient to assess risk.2,3 Bioaerosols, or airborne biological particles from animal operations, pose a less visible but no less significant threat to produce safety. Until recently, producers lacked the tools to quantify that threat at the field level.
Recent advances in biosensing and molecular diagnostics are closing this gap. The work of the Verma Lab at Purdue University has demonstrated a practical and scalable approach to measuring the risk posed by bioaerosol contaminants using the bacterial order Bacteroidales as a quantitative fecal indicator.4 Bacteroidales is a particularly reliable marker of fecal contamination. It is present in feces at high concentrations—comprising 30–40 percent of total fecal bacteria and reaching levels of 10⁹ to 10¹¹ CFU/g—making it significantly more abundant than most pathogens.5 This high prevalence enhances its detectability and ensures a more uniform distribution in contaminated environments.6,7 Additionally, Bacteroidales has a low background presence in non-fecal sources and, as an obligate anaerobe, does not proliferate in ambient environmental conditions.5 These characteristics make it a more specific and reliable indicator of recent fecal contamination.8 Bacteroidales also exhibits strong host specificity. Its 16S rRNA gene contains host-specific markers that can be used for microbial source tracking.9,10 Together, these traits make Bacteroidales a powerful quantitative tool for evaluating fecal contamination risk.
With a simple collection method and both lab- and field-based analytical tools, our approach offers new opportunities for evidence-based decision-making in pre-season and pre-harvest risk assessments (Figure 1).
FIGURE 1. Illustration of the collection flags used for collecting samples, detection of Bacteroidales in the back of a car, and a schematic of potential risk evaluation results (image reproduced from Wang et al.5)
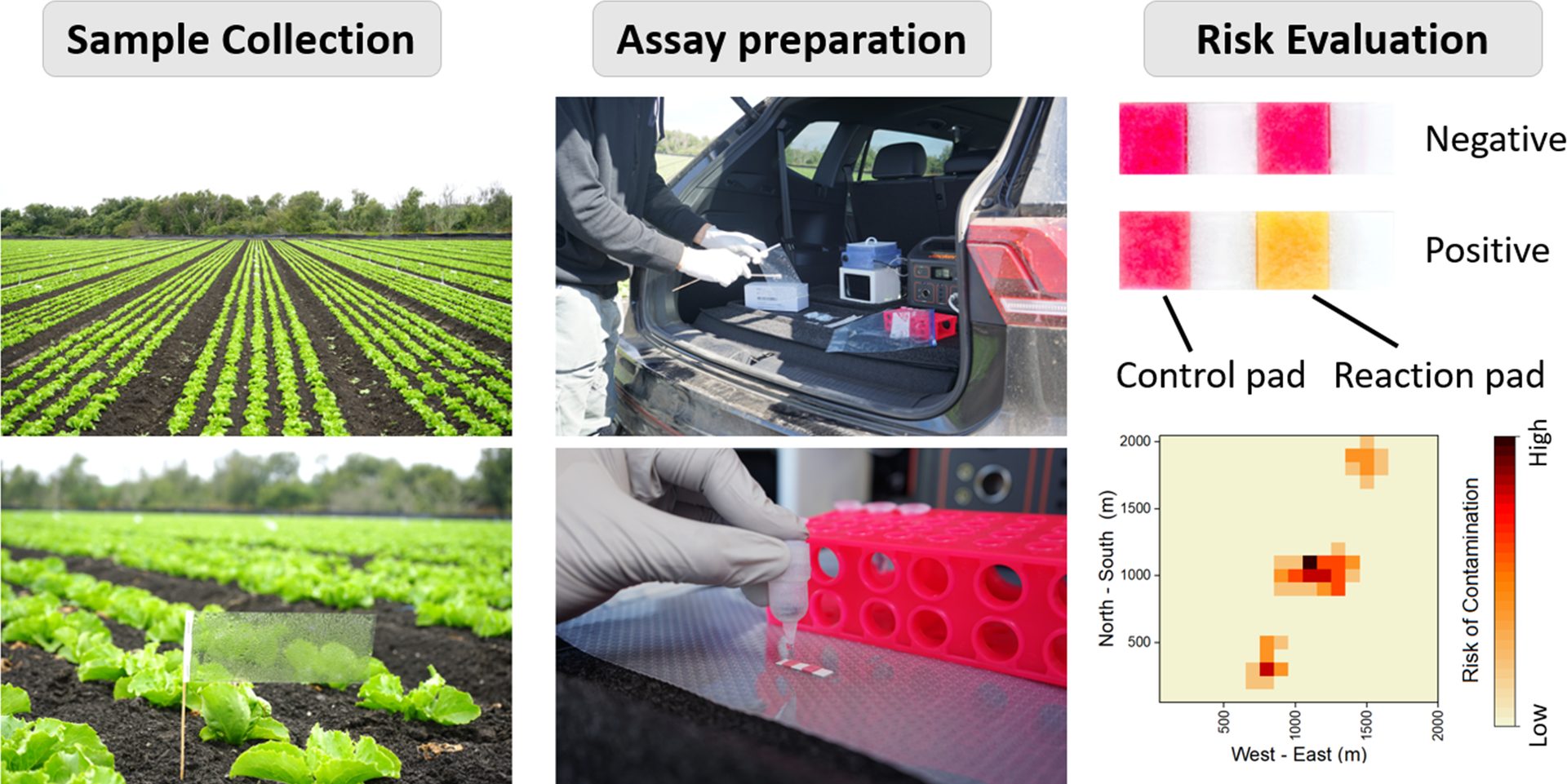
FIGURE 1. Cut Fresh has a number of best practices, auditing, and certification efforts in place to ensure the highest standards of food safety, food quality, and sanitation (Image courtesy of Cut Fresh LLC / Anna Patsakham)

"Our lab developed a sampling method as unassuming as it is effective: a plastic 'collection flag' mounted on a wooden skewer… These flags allow the passive deposition of particles from the air over a period of hours or days."

Turning Invisible Risks into Measurable Data
The LGMAs have long acknowledged the role of bioaerosols in contamination, yet site-specific quantification has remained elusive. Our lab developed a sampling method as unassuming as it is effective: a plastic "collection flag" mounted on a wooden skewer (or a wooden stake for tougher soils).4 These flags allow the passive deposition of particles from the air over a period of hours or days. We swab the surface of these collection flags and then use quantitative polymerase chain reaction (qPCR) to detect and quantify Bacteroidales DNA on the flag surface.
Our findings indicate that background levels of Bacteroidales near well-managed produce fields are low—typically in the range of 0–2 copies per cm².11 In contrast, fields adjacent to animal operations show elevated concentrations, often from 10³–10⁴ copies per cm².4 This 3–4-log difference provides a strong quantitative signal for assessing potential contamination.
Through multiple studies, we have identified a preliminary actionable threshold: approximately 200 copies/cm². At or above this level, we recommend follow-up testing, mitigation efforts, or temporary exclusion of affected areas. This threshold helps bridge the gap between qualitative observations and practical interventions.
From Lab Bench to the Field: Portable Detection with LAMP
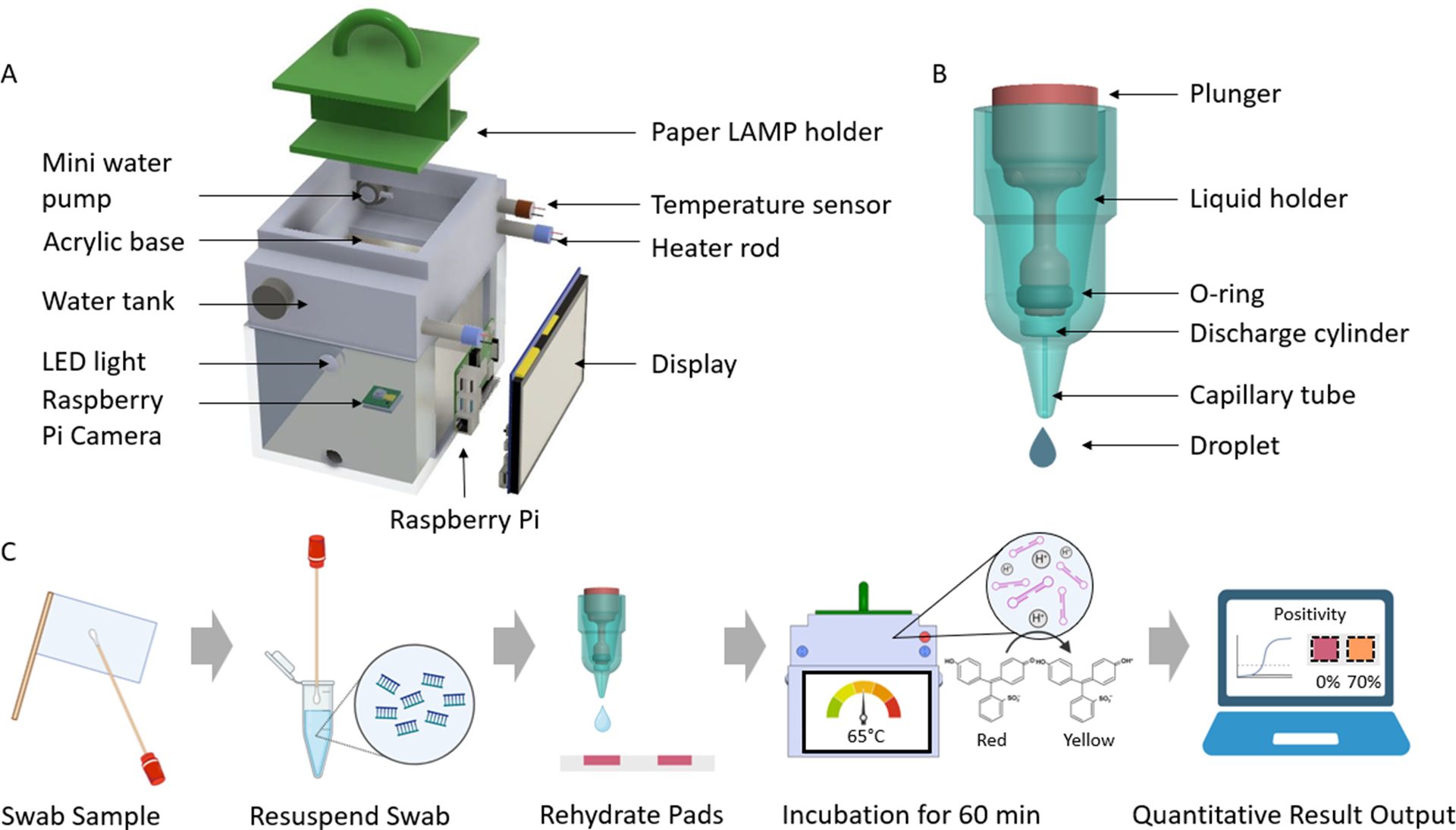
While qPCR is a gold standard for nucleic acid detection, its application in the field is limited by infrastructure, time, and cost. To make fecal contamination assessments more accessible and timely, we developed a portable testing system based on loop-mediated isothermal amplification (LAMP).5 The system, known as Field-Applicable Rapid Microbial Loop-mediated Isothermal Amplification Platform (FARM-LAMP), includes a compact water bath, temperature regulation, an imaging module, and microfluidic paper-based analytical devices (µPADs) (Figure 2).5
FIGURE 2. Design and workflow of the integrated, paper-based LAMP testing platform (image reproduced from Wang et al.5)
LEGEND
(A) Design of FARM-LAMP. Key components of FARM-LAMP include a heating unit, temperature control system, and a high-resolution imaging module.
(B) Design of the drop generator. The drop generator delivers constant 27 µL of sample for the LAMP µPADs.
(C) Overall operation workflow of the platform. The user swabs a collection flag with a sterile swab, following by resuspension of the swab in 200 µL of water. The resuspension is then accurately dispensed on µPADs via the drop generator. Subsequently, µPADs are incubated at 65 °C for one hour, enabling LAMP amplification and a pH-induced color change from red to yellow in the presence of DNA synthesis. Post-reaction, the algorithm analyzes the color of each µPAD to provide a quantitative readout (e.g., sample concentration in copies/cm2).
FARM-LAMP can detect as few as 3 copies/cm² of Bacteroidales and returns results in under one hour (Figure 3).5 Its performance is on par with qPCR, showing 100 percent concordance in side-by-side field trials at commercial lettuce farms and Purdue's Animal Sciences Research and Education Center (ASREC).5
The µPADs use a colorimetric LAMP assay that turns from red to yellow when DNA is amplified (Figure 3).5 Time-lapse imaging and automated analysis yield both qualitative and quantitative data, reducing user subjectivity and eliminating the need for complex lab procedures (Figure 3).5
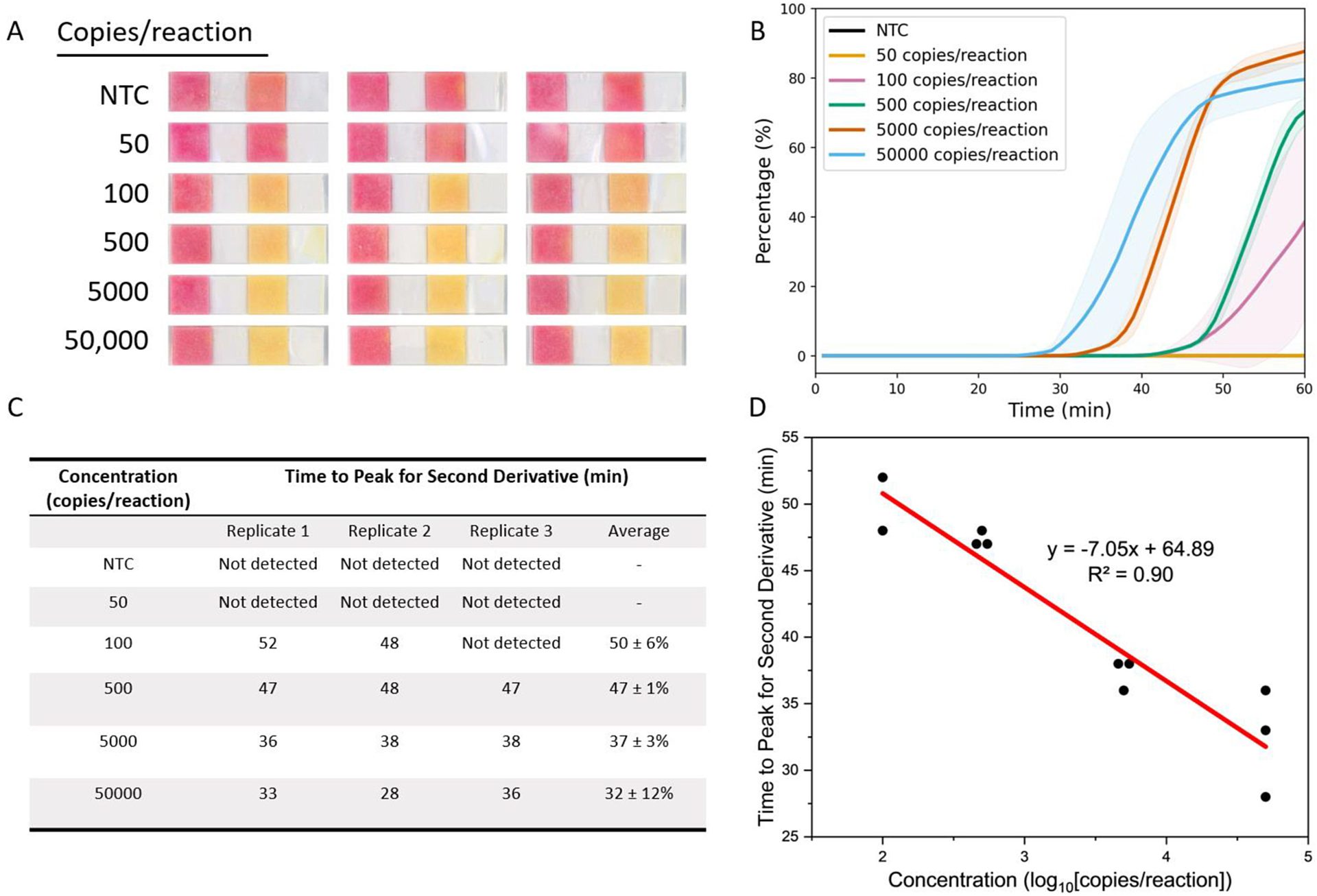
FIGURE 3. Limit of detection (LoD) and quantitative analysis of paper LAMP assays (image reproduced from Wang et al.5)
LEGEND
A) Endpoint (at 60 min.) result of colorimetric LoD on paper using quantified swine stool DNA at the indicated concentration (copies/reaction). The NTC replicates are reactions using nuclease-free water in lieu of DNA.
(B) Quantitative analysis of the LoD experiment in A using the image analysis algorithm. The shaded area represents the standard deviations.
(C) Summary table of time to peak for the second derivative calculated from B. The average of the three replicates was reported as x̄ ± relative standard deviations.
(D) Linear fit analysis generated comparing the time-to-peak for the second derivative (C) and the concentration in logarithmic scaling. A regression line was generated to quantify the concentration of the reaction using the sample's time-to-peak value.
“Each step in the 'rings of defense' must be used consciously and with proper consideration, as it may be obvious what caused the noncompliance, and its resolution may be straightforward, simple, and not costly.”


Integration into Food Safety Protocols
These tools are not meant to replace pathogen-specific testing, but rather to serve as an early warning system. Since Bacteroidales are abundant in feces and exhibit high host specificity, they are a reliable marker of recent fecal contamination—even when pathogens may be present in low or undetectable concentrations.
This risk-based approach aligns with the Food Safety Modernization Act's (FSMA's) emphasis on preventive controls and continuous improvement.12 By quantifying environmental risks in real time, growers can adjust harvest decisions, implement targeted interventions, and document their efforts for regulatory or third-party audits.
The Road Ahead
We are currently expanding the use of a field sampling and analysis service through pilot studies in commercial production environments to refine the approach and validate its value to growers. We are also developing predictive models that integrate weather patterns and geospatial variables to determine the optimal timing and placement of these service assessments. By combining real-time diagnostics with environmental intelligence, we aim to create a proactive system that enables growers to anticipate contamination risks before they impact harvests. These advances will help ensure that fresh produce safety continues to evolve with greater precision, responsiveness, and resilience.
Acknowledgment
This work was funded in part by the Center for Produce Safety (CPS Award Number: 2021CPS12) project, "Field evaluation of microfluidic paper-based analytical devices for microbial source tracking." We acknowledge support from the CPS Award Number: 2023CPS11 project, "Testbeds for microbial source tracking using microfluidic paper-based analytical devices." Any opinions, findings, conclusions, or recommendations expressed in this publication are those of the author(s) and do not necessarily reflect the views of the Center for Produce Safety.
Notes
During the preparation of this work, the author used ChatGPT and Grammarly to organize a draft of the article and check for grammar errors. After using these tools, the author reviewed and edited the content as needed and takes full responsibility for the content.
References
- California Leafy Greens Marketing Agreement (LGMA). "Future Focus." https://lgma.ca.gov/future-focus.
- Arizona Leafy Greens Marketing Agreement (LGMA). "Commodity Specific Food Safety Guidelines for the Production and Harvest of Lettuce and Leafy Greens." August 18, 2020. https://www.leafygreenguidance.com/wp-content/uploads/2020/09/Arizona-GAPS-Metrics-2020-Version-13-FINAL-Aug-2020.pdf.
- California LGMA. Pre-Harvest Testing Guidance. 2021. https://assets.lgma.ca.gov/downloads/Appendix-I-Pre-Harvest-Testing-Guidance-20210416.2_A11Y.pdf.
- Wang, J., M. Ranjbaran, A. Ault, and M.S. Verma. "A loop-mediated isothermal amplification assay to detect Bacteroidales and assess risk of fecal contamination." Food Microbiology 110 (2023): 104173. https://pubmed.ncbi.nlm.nih.gov/36462829/.
- Wang, J., S. Kaur, A. Kayabasi, M. Ranjbaran, I. Rath, I. Benschikovski, B. Raut, K. Ra, N. Rafiq, and M.S. Verma. "A portable, easy-to-use paper-based biosensor for rapid in-field detection of fecal contamination on fresh produce farms." Biosensors and Bioelectronics 259 (2024): 116374. https://pubmed.ncbi.nlm.nih.gov/38754195/.
- Mascorro, J.A., L.G. Hernández-Rangel, N. Heredia, and S. García. "Bacteroidales as Indicators and Source Trackers of Fecal Contamination in Tomatoes and Strawberries." Journal of Food Protection 81 (2018): 1439–1444. https://pubmed.ncbi.nlm.nih.gov/30080121/.
- Ordaz, G., J.A. Merino-Mascorro, S. García, and N. Heredia. "Persistence of Bacteroidales and other fecal indicator bacteria on inanimated materials, melon and tomato at various storage conditions." International Journal of Food Microbiology 299 (2019): 33–38. https://pubmed.ncbi.nlm.nih.gov/30952015/.
- Ravaliya, K., J. Gentry-Shields, S. Garcia, N. Heredia, A. Fabiszewski de Aceituno, F.E. Bartz, J.S. Leon, and L.-A. Jaykus. "Use of Bacteroidales Microbial Source Tracking to Monitor Fecal Contamination in Fresh Produce Production." Applied and Environmental Microbiology 80 (2014): 612–617. https://pmc.ncbi.nlm.nih.gov/articles/PMC3911084/.
- Somnark, P., N. Chyerochana, S. Mongkolsuk, and K. Sirikanchana. "Performance evaluation of Bacteroidales genetic markers for human and animal microbial source tracking in tropical agricultural watersheds." Environmental Pollution 236 (2018): 100–110. https://pubmed.ncbi.nlm.nih.gov/29414329/.
- Zhang, Y., R. Wu, K. Lin, Y. Wang, and J. Lu. "Performance of host-associated genetic markers for microbial source tracking in China." Water Research 175 (2020): 115670. https://pubmed.ncbi.nlm.nih.gov/32171096/.
- Wang, J., M. Ranjbaran, and M.S. Verma. "Bacteroidales as a fecal contamination indicator in fresh produce industry: A baseline measurement." Journal of Environmental Management 351 (2024): 119641. https://pubmed.ncbi.nlm.nih.gov/38064988/.
- U.S. Food and Drug Administration (FDA). "FSMA Final Rule on Produce Safety." Content current as of November 13, 2024. https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-produce-safety.
- California LGMA. "CAFO Assessment Form Pre-Season & Pre-Harvest SOPs." https://assets.lgma.ca.gov/downloads/SOP_CAFOs_PreSeason_PreHarvest_Assessment_Final_A11Y.pdf.
Mohit Verma, Ph.D. is an Associate Professor within Purdue University's Department of Agricultural and Biological Engineering, as well as the Weldon School of Biomedical Engineering. Dr. Verma specializes in biosensors for probing microbiomes at the point of need. His innovative research encompasses a wide spectrum of applications, spanning plants, animals, humans, and the environment. His laboratory pioneers field-deployable biosensors, offering swift characterization solutions for viruses, bacteria, antimicrobial resistance genes, and fungi.

