SCROLL DOWN
Marine Biotoxin Control for Molluscan Shellfish
It is critical to ensure the appropriate control of marine biotoxins to prevent contaminated product from reaching consumers
By Stacey Wiggins, Ph.D., and Erika Anderson, M.S.
Video credit: Esperanza33/Creatas Video via Getty Images
> CATEGORY
Algae form the base of the aquatic food web, given their significant contribution to global photosynthesis. Algae are plant‐like life forms that float or move on their own in water. They vary in size from very small (microalgae; i.e., phytoplankton) to very large (macroalgae; e.g., seaweed). This article focuses on microalgae and the small percentage that may be harmful. A rapid increase in the population of algae, called an algal bloom, can deplete oxygen in the water column, creating hypoxic or anoxic conditions. These conditions can lead to changes in food web dynamics and, therefore, changes in the species of marine life present; reduce light penetration through the water column; and cause fish kills, generally by the clogging of the gills.
A subset of these harmful microalgae is known as toxic algae. The toxins (poisons) produced by toxic algae in the marine environment are referred to as marine biotoxins; however, biotoxins may also be produced by certain freshwater algae and cyanobacteria. This article focuses on the toxins in the marine environment that may accumulate in molluscan shellfish such as oysters, clams, and mussels. If shellfish contaminated with these toxins are consumed, the toxins can sicken, or in extreme cases prove fatal, to the people who eat them.1 Given the potential adverse effects on human health, it is critical to ensure the appropriate control and management of marine biotoxins to prevent contaminated product from entering interstate commerce.
These toxins are not destroyed by cooking, freezing, or other food preparation processes, which highlights the importance of seafood safety programs that address understanding of the sources and fate of marine biotoxins, as well as control and management strategies.1 U.S. Food and Drug Administration's (FDA's) Center for Food Safety and Applied Nutrition (CFSAN) is responsible for ensuring that the nation’s food supply is safe, and in this article we focus on the control of marine biotoxins to prevent contamination of molluscan shellfish. We will address the sources, risk, and management of marine biotoxins found in molluscan shellfish and methods to identify contaminated shellfish meat before marketing.
Molluscan shellfish (clams, mussels, oysters, and whole or roe-on scallops) fall under the purview of the National Shellfish Sanitation Program (NSSP).2 The NSSP is a federal-state cooperative program that includes representatives from shellfish-producing and non-producing states, FDA, the Environmental Protection Agency (EPA), the National Oceanic and Atmospheric Administration (NOAA), and the shellfish industry. The NSSP is recognized by FDA and the Interstate Shellfish Sanitation Conference (ISSC)3 as the standard for the safety and sanitary control of molluscan shellfish intended for human consumption in interstate commerce.
The ISSC holds routine meetings, typically every two years, to deliberate new proposals aimed at updating and refining program requirements. The requirements for molluscan shellfish safety are documented in the NSSP Guide for the Control of Molluscan Shellfish. Due to the COVID-19 pandemic, the last ISSC meeting was held in 2019; therefore, the most current version of the Guide is the 2019 Revision.4 The Guide includes the purpose and definitions, model ordinance (which states may adopt into regulation), public health reasons and explanations, guidance documents, suggested forms, NSSP policy-setting documents, shellfish federal regulations, FDA manual of interpretations, and the history of the NSSP. The authors will highlight the marine biotoxins covered in the Guide and associated requirements. For more detail on marine biotoxin management requirements, see Section II, Chapter IV @ .04. Additional details and laboratory methods are found in Section IV, Chapter II, .02 and .14.
Toxins Sources and Geographic Distributions
The Guide explicitly covers five main intoxication syndromes that may be caused by marine biotoxins and establishes threshold concentrations at or above which shellfish growing areas must be placed in the closed status, meaning that harvesting is prohibited. The following are the syndromes and toxin thresholds:
- Paralytic shellfish poisoning (PSP): 80 µg saxitoxin-dihydrochloride equivalents/100 g
- Amnesic shellfish poisoning (ASP): 2 mg domoic acid/100 g
- Neurotoxic shellfish poisoning (NSP): 20 MU/100 g
- Diarrhetic shellfish poisoning (DSP): 0.16 mg okadaic acid equivalents/kg
- Azaspiracid shellfish poisoning (AZP): 0.16 mg azaspiracid-1 equivalents/kg.
Knowing the sources and geographic distributions of the toxins causing these syndromes is vital to implementing appropriate controls and preventing the harvest and sale of contaminated molluscan shellfish.
PSP toxins, also referred to as saxitoxins, are primarily produced by certain microalgae, classified as dinoflagellates, which belong to the Genus Alexandrium5 (Figure 1).The dinoflagellate species Pyrodinium bahamense is also a PSP toxin producer often found off the coast of Florida and throughout the Caribbean. Saxitoxin is one of numerous toxins that comprise the PSP toxin family. Symptoms of PSP include tingling of the lips, mouth, and tongue; numbness of the extremities; weakness; nausea; shortness of breath; dizziness; and respiratory paralysis.1 PSP-producing algae have been documented off the U.S. northeast and west coasts, including Alaska, and the coast of Florida.6
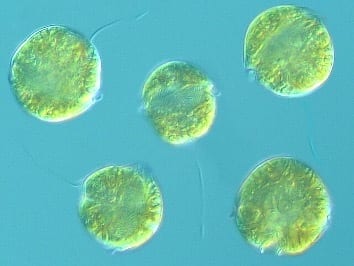
FIGURE 1. A Dinoflagellate Belonging to the Genus Alexandrium, Known to Produce Paralytic Shellfish Poisoning Toxins
“Like many other sectors, the meat and poultry industry has faced unprecedented disruption from the COVID-19 pandemic.”


ASP toxin, or domoic acid, is produced by certain microalgae referred to as diatoms of the genus Pseudo-nitzschia.7 Toxic versus non-toxic species are difficult to distinguish using standard monitoring techniques such as light microscopy. Confirmation of toxic species may require specialized microscopic or molecular techniques. ASP symptoms include vomiting, diarrhea, abdominal pain, confusion, and short-term memory loss.1 ASP-producing algae have been documented to occur off the U.S. northeast and west coasts and the coast of Florida.6
NSP toxins, referred to as brevetoxins, are produced by the dinoflagellate Karenia brevis.8 Numerous toxins make up the brevetoxin family. Furthermore, shellfish may metabolize the toxins into different forms, which also contribute to toxicity. Symptoms of NSP include tingling and numbness of the lips, tongue, and throat; muscular aches; dizziness; diarrhea; and vomiting.1 Blooms of Karenia brevis are typically restricted to the Gulf of Mexico and the coast of Florida.6 Unlike the other toxins discussed in the Guide, brevetoxins also affect people through inhalation of aerosols generated during toxic blooms, often called "red tides."
DSP toxins include okadaic acid and dinophysistoxins.9 Dinophysis and Prorocentrum are two types of dinoflagellates that may produce these toxins. The DSP toxin family comprises multiple toxins, and shellfish tend to add fatty acid esters to the toxins upon ingestion. These metabolites are not toxic in mouse bioassays but have been shown to contribute to illness. DSP symptoms include nausea, vomiting, diarrhea, abdominal pain, chills, headache, and fever.1 DSP toxin producers have been found off the U.S. east and west coasts and in the Gulf of Mexico.6
AZP is caused by azaspiracids, produced by very small dinoflagellates in the Genus Azadinium.10 Due to their small size, it is difficult to distinguish toxic from nontoxic species in this group using a standard microscope. AZP symptoms include nausea, vomiting, stomach cramps, and diarrhea.1 Closures of shellfish growing areas in the U.S. due to AZP toxins have not been necessary to date. The only illnesses documented in the U.S. were attributed to imported product. AZP toxin producers have not been identified as a problem off the coasts of the U.S., but remain an emerging issue to better understand the presence and toxin production of Azadinium in the U.S.
Marine Biotoxin Control
In accordance with the Guide, marine biotoxin management plans are required for growing areas that have been impacted in an illness outbreak, or where toxic phytoplankton have been documented to occur and where toxins are prone to accumulate in shellfish. Marine biotoxin contingency plans, however, are required for growing areas where illness outbreaks have not been implicated or where toxin-producing phytoplankton have not been documented historically. Marine biotoxin contingency plans, as the name implies, address the unforeseen management of emerging toxins that are either new or present in a new location.
Requirements of both plans include initiating an emergency sampling, closing growing areas, embargoing shellfish, preventing the harvest of contaminated species, providing for a product recall, disseminating information about the event, coordinating control actions taken by authorities and federal agencies, and establishing criteria for reopening growing areas once they have been placed in the closed status. Additionally, marine biotoxin management plans require maintaining a toxin-producing phytoplankton and/or shellfish sampling program and ensuring that all shellfish harvested from growing areas or portions of growing areas that are placed in the controlled access status meet all conditions of harvest restrictions prior to being entered into commerce.
Five marine biotoxin management strategies are available for maintaining a toxin-producing phytoplankton and/or shellfish sampling program. Regardless of the strategy chosen, minimum sample numbers are required. The baseline database for informing biotoxin management plans must include at least 36 samples over the course of at least three years. This should be established for each growing area or for hydrographically linked water bodies.
The five management strategies are:
- Phytoplankton monitoring: Routine monitoring of phytoplankton, whereby established concentrations would trigger subsequent testing for toxins in shellfish meat (Figure 2).
- Shellfish toxicity monitoring: Routine monitoring of toxins in shellfish meat, either species-specific or via use of a high-risk species as an indicator for other shellfish species.
- Pre-harvest testing: Toxin testing of shellfish meats in advance of harvest from the intended harvest area, the results of which are valid for a short period of time.
- Shellfish meat lot testing: Toxin testing of shellfish meats after harvest, whereby controlled access requirements (e.g., holding of product until test results demonstrate that the product is safe) apply.
- Pre-harvest screening coupled with lot testing: Toxin screening of shellfish meats from the intended harvest area, whereby acceptable screening results are needed to initiate the harvest of shellfish. Lot testing of shellfish meats is subsequently performed upon landing, in conjunction with adherence to controlled access requirements.
Controlled access status is a new growing area status option. Rather than placing the shellfish growing area in the closed status, controlled access status is applied to allow harvesting in areas with biotoxin concerns where routine monitoring or pre-harvest testing are not practical. For growing areas where the controlled access status is employed, the State Shellfish Control Authority determines the additional requirements necessary to ensure the safe harvest of product. Controlled access status is a designation of an approved area or a conditionally approved area in the open status. Additional requirements shall be included in harvest permit conditions. All shellstock harvested from growing areas in the controlled access status shall be tagged with restricted shellstock harvest tags.

FIGURE 2. Phytoplankton Monitoring as a Marine Biotoxin Management Strategy
Laboratory Methods
The NSSP has designated "approved" and "approved limited use" methods for use in support of the program. Approved methods are considered primary or core methods and are sometimes referred to as reference methods. While some methods are deemed fit-for-purpose based on historical use or peer review, the ISSC Laboratory Committee now relies on single laboratory validation to determine the suitability of the method for specific purposes.
Approved limited use methods are approved for a very specific application. The reasons for the limitations vary, but generally these tests are new, alternative, or screening methods. For example, several of these methods are approved limited use to make precautionary closures of growing areas but cannot be used to reopen an area. Like approved methods, approved limited use methods are evaluated for specific applications and determined to be fit-for-purpose through single laboratory validations.
Approved methods include:
- Mouse bioassay (MBA) for NSP and PSP toxins: The exact MBA for PSP and NSP toxins differ, however, with the NSP mouse bioassay taking much longer and having lower throughput than the bioassay for PSP.
- Receptor binding assay for PSP toxins in mussels: Like the MBA, this assay does not differentiate which toxins are present; rather, it yields a collective response for overall toxicity, which is desirable when multiple toxins—each with a different toxicity—are present as with PSP toxins.
- Post-column oxidation (PCOX) HPLC method for PSP toxins in mussels, clams, oysters, and scallops: This method, requiring a specialized instrument and an experienced analyst, provides the identification and concentrations of each of the toxins that make up the PSP toxin family. The analyst must calculate an overall toxicity in saxitoxin equivalents.
- Liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) method for DSP toxins in clams: LC-MS/MS requires a specific instrument and an experienced analyst and allows for the separation, identification, and quantitation of multiple toxins. The mass of each toxin is measured, thereby confirming the results.
- HPLC with ultraviolet detection (HPLC-UV) for ASP toxin detection: As with all HPLC methods, one challenge is to ensure that a toxin is being measured, and not an interfering compound.
Approved limited use methods include:
- Abraxis shipboard ELISA, an enzyme-linked immunosorbent assay, for PSP toxins for specific use as a field kit as the onboard or pre-harvest screening component of the pre-harvest screening with lot testing strategy. A limitation is that the extraction must be performed using 70% isopropanol and 5% acetic acid in a 2.5 to 1 ratio.
- Scotia Rapid Test, when performing the extraction with boiling hydrochloric acid, is used for screening PSP toxins in shellfish. This method can be used only to make a precautionary closure, maintain an area in an open status, or determine when to conduct a mouse bioassay. It is a lateral flow device, similar to a pregnancy test, and gives a positive or negative result that is determined visually.
- The Neogen Reveal 2.0 ASP kit for domoic acid in shellfish is also a lateral flow device, but positive or negative results are scored using an automated reader, thereby eliminating the need for an analyst to determine the result visually. It can be used for making precautionary closures of growing areas.
- The Receptor binding assay for PSP toxins, described above, can also be used for whole or roe-on scallops or clams, specifically for making growing area closures.
- MARBIONC ELISA is used for NSP toxins for oysters, hard clams, and sunray venus clams. A negative result can substitute for testing by an approved method for the purpose of controlled relaying, controlled harvest end-product testing, or to reopen a previously closed area. A positive result requires additional testing by an approved method or to support the same management decisions as samples failing by an approved method.
Overall, successful marine biotoxin control relies on the most up-to-date science, on the spatial and temporal distributions of toxic algae and/or toxins in molluscan shellfish, and on the appropriate use of laboratory methods as defined in the most current revision of the Guide. All of these strategies must be reflected in comprehensive marine biotoxin management and contingency plans.

FIGURE 3. FDA's Online Learning Module, "Marine Biotoxin Management for Molluscan Shellfish"
Marine Biotoxin Management Online Learning Module
FDA has recently developed an updated online learning module (Figure 3) covering marine biotoxin management requirements for molluscan shellfish intended for interstate commerce. This interactive training module provides detailed information on the current marine biotoxin requirements, as described in the NSSP Guide for the Control of Molluscan Shellfish: 2019 Revision.
The video, which encompasses three learning modules, is a tool to help the seafood industry and state and federal agencies understand biotoxin sources and geographic distributions, develop or improve marine biotoxin management and contingency plans, and determine the appropriate laboratory methods for specific biotoxin applications.
The online learning module can be accessed at www.fda.gov/seafood.
References
- U.S. Food and Drug Administration. Bad Bug Book: Foodborne Pathogenic Microorganisms and Natural Toxins. 2nd Ed. 2012. https://www.fda.gov/media/83271/download.
- U.S. Food and Drug Administration. "National Shellfish Sanitation Program (NSSP)." October 29, 2020. https://www.fda.gov/food/federalstate-food-programs/national-shellfish-sanitation-program-nssp.
- Interstate Shellfish Sanitation Conference. "ISSC." 2022. https://www.issc.org/.
- U.S. Food and Drug Administration. NSSP Guide for the Control of Molluscan Shellfish: 2019 Revision. 2019. https://www.fda.gov/media/143238/download.
- Etheridge, S.M. Toxicon 56 (2010): 108–122.
- U.S. National Office for Harmful Algal Blooms. "Distribution of HABs in the U.S." Wood Hole Oceanographic Institute 2019. https://hab.whoi.edu/maps/regions-us-distribution/.
- Lefebvre and Robertson. Toxicon 56 (2010): 218–230.
- Watkins et al. Marine Drugs 6, 3 (2008): 431–455.
- Trainer et al. Marine Drugs 11, 6 (2013): 1815–1835.
- Salas et al. Harmful Algae 10, 6 (2011): 774–783.
Stacey Wiggins, Ph.D., is Science Advisor at FDA's Office of Food Safety's Division of Seafood Safety, located within FDA's Center for Food Safety and Applied Nutrition.
Erika Anderson, M.S., is Senior Instructional Systems Specialist at the Office of Management of the Staff College for FDA's Center for Food Safety and Applied Nutrition.
