BACK TO BASICS
By Nina Da Costa, P.G.Dip., IDipNEBOSH, Consulting Food Safety Expert
Critical Implications of Hygienic Zoning for Food and Beverage Processing
Hygienic zoning is the invisible map of barriers and boundaries that help prevent contaminants from entering food products

Image credit: gerenme/E+ via Getty Images
SCROLL DOWN
Hygienic zoning (HZ) is a food safety hazard control strategy applied in food and beverage manufacturing and processing environments. Its primary objective is to prevent the spread of food safety hazards—microbial (pathogens), chemical (allergens), and physical (foreign bodies)—from dirty (low-risk) to clean (high-risk) areas, and finally into the finished food product. The final product may be a raw material for further processing by industry or a ready-to-eat (RTE) food with no kill step.
The term "areas" encompasses food contact surfaces, primary packaging materials, equipment, facility infrastructure, food and non-food environments, tools, and the food itself—all of which may become contaminated and/or contribute to contamination. Personnel are a critical vector, as they interact directly with food and food contact zones.
HZ involves segregating areas of a facility into zones, grading them according to product contamination risk levels, and applying targeted controls informed by regulations, standards, facility hygiene needs, processing activities, environmental conditions, and available technologies. HZ can effectively prevent contamination of food at various stages of handling before it reaches the finished product.
HZ is a foundational prerequisite for the design or redesign of food premises and must be correctly applied before critical decisions relating to facility layout, airflow management, personnel pathways, and equipment installation are made. It is intended to prevent contamination from spreading across zones while microbial multiplication in the product is managed by process controls (e.g., pasteurization, chilling, acidification, etc.). Together, HZ and process control create a complementary system that reinforces product safety.
Core Elements of Hygienic Zoning
HZ is based on guiding principles that establish boundaries to prevent hazards from reaching the higher‑risk zones and contaminating the final product through vectors or contamination pathways such as water, air, people, equipment, tools, the building/facility, and waste. The two main pillars of HZ for disrupting these vectors are barriers and routines. Barriers are the engineered or physical separations that enforce zone integrity, while routines are the behavioral disciplines and practices that sustain it. Core elements of HZ are outlined in Table 1 below.
TABLE 1. Core Elements of Hygienic Zoning (Credit: N. Da Costa)
Risk Assessment and Zone Classification
Risk assessment is an analytical approach for HZ involving hazard identification and a hazard evaluation, followed by risk scoring. The risk score, developed using a pre-defined risk matrix, determines the basis for zone classification (high-risk/medium-risk/low-risk).
Most certification standards (e.g., BRCGS, SQF, FSSC 22000, Codex HACCP) require documented risk assessments (for hygienic zoning or as part of a HACCP plan). Some, like BRCGS, have their own, unique methodology for zone classification. Establishing an effective and acceptable methodology for deployment is necessary to demonstrate compliance.
The main logical steps of a risk assessment are shown in Table 2.
TABLE 2. Risk Assessment Steps for Hygienic Zoning (Credit: N. Da Costa)
Overview of Risk Zones and Hygienic Zoning Requirements
Several certification standards—including GFSI schemes, corporate models, and industry guides—mandate or necessitate the hygienic zoning approach to determine the risk zones/hygienic zones for preventing the spread of contamination into higher-risk zones and the final product.
Formal Hygienic Zoning Models: BRCGS Global Standard Food Safety
BRCGS' Global Standard Food Safety (Issue 9)1 has clear-cut criteria (Appendix 2) for sites to assess the production risk zones (hygienic zones) for the products manufactured, processed, or packed at the site. These are mandatory for third-party certification (Table 3).
TABLE 3. Risk Zones—BRCGS Global Standard Food Safety (Issue 9) (Credit: BRCGS and N. Da Costa)
Corporate Hygienic Zoning Models
Global corporate models like that of Cargill2 clearly differentiate between hygiene zones located inside the facility and technical zones located outside the facility. This ensures global consistency for the company's sites and focuses resource allocation where it matters the most.
Hygienic Zoning for Smaller and Less Developed Businesses
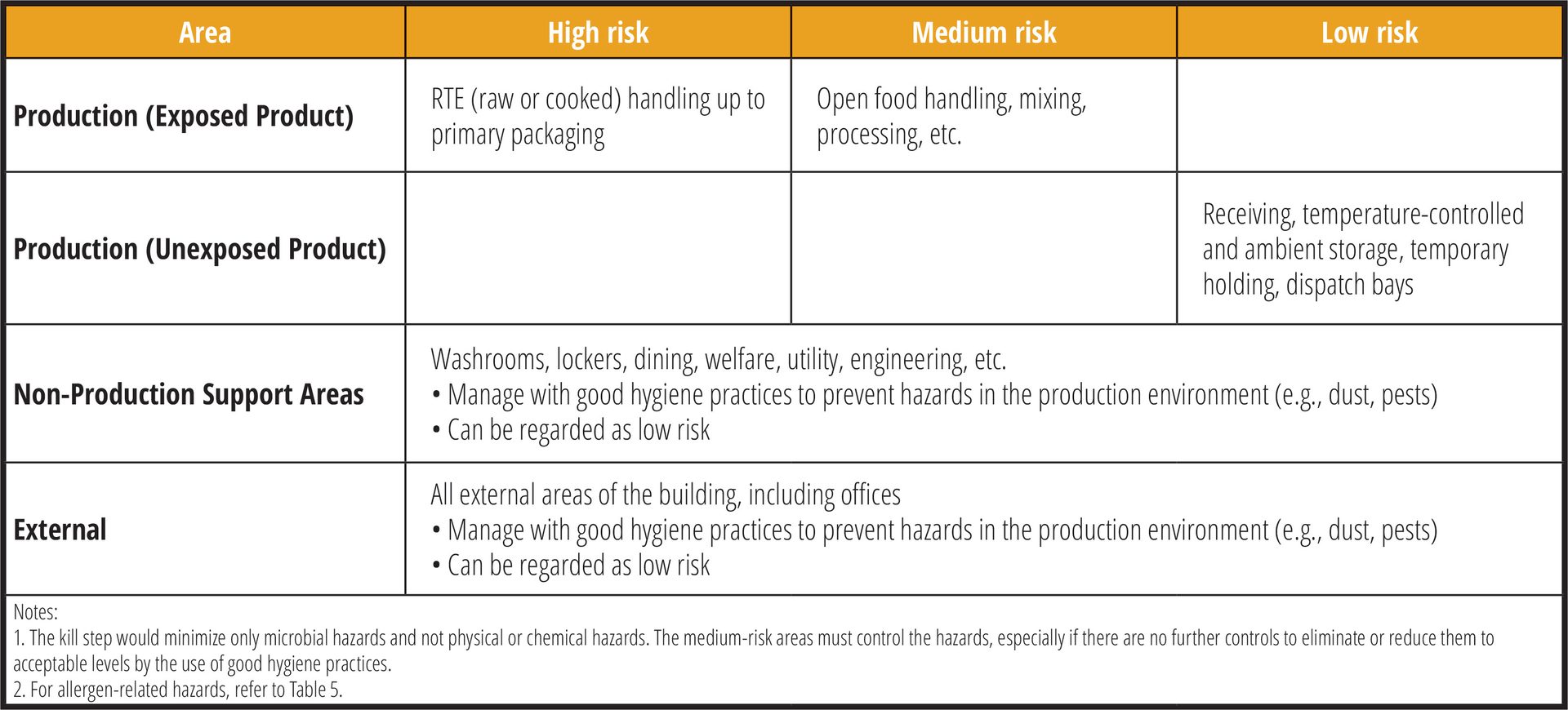
Where acceptable from a legal perspective or otherwise, smaller businesses may choose to adopt a simpler, more straightforward approach (Table 4). In some instances, the kill step may not apply (e.g., slicing of ready purchased cold cuts, preparing raw salads, etc.).
TABLE 4. A Simplified Hygienic Zoning Model (Credit: N. Da Costa)
Allergen-Related Hygienic Zoning Considerations
Allergen‑related hygienic zoning must be integrated into the overall hygienic zoning approach to prevent cross‑contamination between allergen and non‑allergen products. Unlike microbial hazards, which can be reduced or destroyed using process controls (e.g., pasteurization), or physical hazards, which can be detected and screened out (e.g., sieving, filtration), allergens are proteins and cannot be removed or neutralized. The only controls for allergens are prevention and segregation, which are essential parts of hygienic zoning (Table 5). Products contaminated with allergens must be segregated and re-labeled for allergen declaration or destroyed.
TABLE 5. Allergen-Related Hygienic Zoning (Credit: N. Da Costa)
Transition Areas
Transition areas are buffer zones that separate two zones of differing risk levels. They serve as controlled environments (airlocks) that enforce the hygiene barrier, ensuring that personnel, equipment, or materials moving between zones do not compromise segregation or introduce hazards.
The design and components of transition areas vary based on the purpose and the zones being separated. Typical components may include airlock arrangements that prevent the entry of unfiltered or contaminated air into clean zones, personal hygiene stations, sanitation systems, inspection points for tools/equipment, systems for controlled transfer of materials, and others.
The risk level of transition areas is dependent on the zones that they separate. This must be documented as part of hygienic zoning risk assessments.
Interrupting the Vectors: Minimizing Hazard Transfer
The vectors identified during the risk assessment must be controlled by the application of barriers and routines. The experience-based risk factors outlined below address the major pathways by which hazards can spread across zones, rather than how a product itself may become contaminated.
Water
- Overhead condensation dripping onto the exposed product or surfaces due to insufficient drying time after wet cleaning
- Pooling water under machinery, uncontrolled leaks, or standing water in waste areas tracked across floors or into production zones by personnel or equipment
- Contamination from hoses due to cross-use and/or hose ends left on drains or on dirty floors between use
- Aerosols contaminating RTE food due to wet cleaning during open food handling, poor physical barriers, order of cleaning from dirty to clean areas, or HVAC systems left running during pressurized wet cleaning
- Aerosol contamination from open drains or wash water due to proximity of food trolleys or workstations near drains
- Ice for RTE food handling picked up from ice machines shared across zones.
Air
- Shared HVAC systems across zones
- Poorly sealed or ventilated transition zones (Table 6)
- Reversal of positive pressure allowing dirty air into clean rooms (e.g., improper balancing of supply vs. return air in the HVAC, building envelope issues like damaged seals around doors, etc.)
- Filter blockages, damaged or loose-fitting filters, fan malfunction, blocked vents, etc.
- Poor preventive maintenance
- Plant extensions without a suitable risk assessment on current system capacity.
TABLE 6. Air Filtration and Ventilation Standards for Hygienic Zoning Compliance (Credit: N. Da Costa)
People
- Shared traffic routes for food and non-food personnel, including visitors
- Transition zones are crowded during peak periods (e.g., shift start), creating a risk of bypassing personal hygiene protocols or of lower capacity
- Assigning food handling tasks to cleaners or food handlers of lower-risk zones
- Poor control of human traffic on wet floors
- Glove use and change, footwear integrity, and cleanliness are poorly managed
- Incompetence and general unawareness of operational "hot spots" where rules are consistently broken.
Equipment
- Shared equipment between zones of differing risk level
- Equipment movement over wet floors (forklifts, pallet jacks, dollies, mobile racks, etc.)
- Lack of wheel disinfection for wheeled equipment moving across zones
- Contaminated aerosols from poorly cleaned foggers and humidifiers harboring biofilms
- Shared washing/cleaning stations for dismantled equipment parts from different zones
- Lack of insulation integrity (e.g., chilled lines, tanks, ducts, etc.) promoting condensate drip.
Tools
- Shared use of cleaning tools across zones or lack of identification (e.g., color coding)
- Shared use of measurement or cutting tools across zones (e.g., cutters, weighing scales, etc.)
- Use of the same tool for two incompatible activities in the same zone (e.g., using pliers for lifting a drain cover for cleaning and then using it to tighten a clamp on the food processing machine)
- Using the same set of tools for dirty and clean areas (e.g., use of screwdriver to adjust a belt guard on a conveyor located near the waste collection area, and later to tighten a fastener on a hopper feeding RTE product in the high‑care zone)
- Lack of control on the use of personal tools, pens, clipboards, pocket thermometers, etc. by personnel conducting inspections and internal audits across multiple zones.
Building/Facility
Failure in the hygienic design of buildings can facilitate transfer of contaminants across the zones and across the facility. This can also cause other vectors to be involved in the spread, namely:
- Pooled water spreading by traffic due to damaged floors or floors not sloping toward drains
- Shared drainage between high- and low-risk zones without adequate controls (e.g., backflow prevention)
- Lack of physical barriers, especially in high-risk areas, or waste docks located close to production
- Unsealed penetrations (e.g., pipes, cables, etc.) passing through walls that separate zones with gaps around them
- Shared doors between zones.
Waste
- Shared waste containers across zones and poor cleaning on return
- Waste storage, collection, or transfer in open bins (aerosols and pest issues)
- Waste collected in bags during bin shortages and dragged across floors
- Waste bins wheeled over pooled water and/or lack of wheel disinfection protocols
- Accumulating recyclables in production areas (airborne fibers, dust, and mold spores).
“While the hygienic zones are identified to prevent contaminants from reaching the clean areas, the FDA Listeria EMP zones are intended for environmental monitoring.”

Reassessment of Hygienic Zoning
While a hygienic zoning review is conducted at planned intervals, a reassessment is initiated in response to a specific trigger that may compromise the integrity of the hygienic zoning suitability.
Common triggers include:
- Process or product changes
- Introduction of new products
- Change in kill step
- Changes in facility layout with infrastructure renovations or expansions
- Operational changes (e.g., increased staffing)
- Outsourcing of activities, customer complaints, recalls, or audit findings
- EMP failures or pathogen detection
- Changes in regulations or standards.
Environmental Monitoring Programs
EMPs are proactive and risk-based surveillance programs designed to routinely sample and test the food production environment for pathogens or hygiene indicators. They are used to verify the effectiveness of the plant sanitation and the hygienic zoning controls. Pathogens of concern in the food environment include Listeria monocytogenes, Salmonella, Escherichia coli, and others.
The U.S. Food and Drug Administration (FDA) recommends that the plant be characterized in terms of a zone system for the purpose of collecting and testing environmental samples for the presence of Listeria spp. (Table 7). Swabs and samples are collected based on a sampling schedule, and the results are tracked over time to enable corrective actions.
Zone 1 (food contact surfaces) has the highest contamination risk and is considered as the primary pathogen control area, necessitating the highest levels of control. Zone 2 (adjacent surfaces) serves as a buffer and signals a risk to Zone 1; it is often treated as a part of primary pathogen control monitoring. Zones 3 and 4 (floors, drains, and external areas) are sentinel zones. Non-compliant samples in Zones 3 and 4 signal that the pathogen could move inward. Sampling across zones helps track pathogen movement and directs the required controls.
While the hygienic zones are identified to prevent contaminants from reaching the clean areas, the FDA Listeria EMP zones are intended for environmental monitoring. Hygienic zones and EMP zones complement each other to maintain product integrity.
TABLE 7. FDA Listeria EMP Zones (Credit: FDA3)
Contextual Overview: Regulations, Standards, and Industry Guidelines
With the rise in pathogen‑related food recalls and evolving regulations, the need for robust hygienic zoning has become undeniable. Several GFSI‑recognized certification schemes, along with HACCP‑based food safety approaches, legal frameworks, and industry guides, explicitly document or implicitly require hygienic zoning as a foundational element in the food and beverage industry.
Significant standards and regulatory frameworks requiring or implying hygienic zoning include:
- FDA's FSMA Final Rule on Requirements for Additional Traceability Records for Certain Foods (2025 update) has extended food traceability for all food and beverage companies to 2028.
- Canadian Food Inspection Agency (CFIA) implies hygienic zoning for Safe Food for Canadians Regulations compliance, especially for high-risk foods.
- Several GFSI schemes and global standards (e.g., FSSC 22000, BRCGS, etc.) include or imply hygienic zoning as an auditable prerequisite program (PRP) requirement.
- ISO 22002–1:2025, Prerequisite Programs on Food Safety—Part 1: Food Manufacturing, is to be applied in conjunction with ISO 22002–100:2025, Prerequisite Programs on Food Safety—Part 100: Common Requirements. Together, they align to establish hygienic zoning as a risk-based requirement and to integrate it with EMPs.
FDA's Food Safety Modernization Act (FSMA), through its Final Rule for Preventive Controls for Human Food, requires facilities to implement EMPs that are appropriate to the food, facility, and hazards identified. Within this framework, the zone concept (Zones 1–4) is widely adopted as a best practice for structuring EMPs, and hygienic zoning is a supporting mechanism to meet EMP obligations.
In summary, hygienic zoning is no longer just a best practice, but a legal expectation across North America.
References
- BRCGS. Global Standard for Food Safety (Issue 9). August 1, 2022. https://www.brcgs.com/product/global-standard-food-safety-issue-9/p-13279/.
- Blücher Group. "Zoning Strategies in Cargill's Food Production Facilities." December 2024. https://www.blucher.com/blog/zoning-strategies.
- U.S. Food and Drug Administration (FDA). Draft Guidance for Industry: Control of Listeria monocytogenes in Ready‑to‑Eat Foods. January 2017. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/draft-guidance-industry-control-listeria-monocytogenes-ready-eat-foods.
Nina Da Costa, P.G.Dip., IDipNEBOSH is a third‑party systems lead auditor, consultant, and trainer. Her technical domains include food safety, food quality, health and safety, environment and sustainability, and operations management. Nina is an accomplished thought leader, technical speaker, author, and subject matter expert with global experience in mentoring businesses and professionals.

