Regulatory Report
By Shruti Nikam, M.S., PCQI, HACCP, SQF, Food Safety and Quality Assurance Manager
Closing the Transparency Gap: FSQA Strategies for Navigating Natural Flavor and Color Regulations
An action plan to validate "natural" claims, maintain supplier transparency, and minimize litigation risk by interpreting changing requirements

Image credit: Inna Reznik/iStock/Getty Images Plus via Getty Images
SCROLL DOWN
Let us consider two identical molecules: one derived from a fruit and the other from a petroleum base. Should both be called "natural?" Take, for example, The Coca-Cola Company's Fanta Berry beverage, which was labeled as containing "100 percent natural flavors" while using DL-Malic acid synthesized from petroleum.1 This exemplifies a commonplace problem in natural flavor labeling; customer expectations often contradict with ingredient realities. Following incidences like these, in April 2025, the U.S. Food and Drug Administration (FDA) announced that it would phase out petroleum-based synthetic dyes by the end of 2026.2
This move indicates a growing need for food safety and quality assurance (FSQA) teams to emphasize rigorous analytical verification, deeper supplier transparency, and robust documentation to ensure that product labels match ingredient composition. These are critical steps in risk management and brand integrity in the face of growing regulatory scrutiny.
Periodic revisions in policies reflect the compounding worries of the public regarding the safety and transparency of food commodities. As regulations continue to change and consumers call for cleaner labels, transparency gaps persist related to regulatory uncertainty and the limitations of analytical methods. This article explores the evolving science, the gaps in regulation, and the food safety and quality strategies needed to safeguard brands, achieve compliance, and develop the next generation of transparent food systems.
Navigating the Transition to Natural Colors and Flavors
Across the U.S., regulatory activity around the use of synthetic colors and flavors is increasing. As regulations change at the state and federal levels, manufacturers are tasked with navigating formulation impediments and ensuring product quality, consistency, availability, and consumer demand. The intention of manufacturers is apparent; reformulation is the new truth and not an option.
While reevaluating synthetic colors and flavors, businesses often re-evaluate their preservatives, emulsifiers, and stabilizers. By investing in stabilizing technologies, companies can achieve enhanced efficiency and coordination beyond flavor and appearance. Laboratories must develop methods like high-performance liquid chromatography (HPLC) for detecting novel targets, such as breakdown products derived from natural pigments or specific markers in flavors that indicate authenticity.
However, reformulation can throw a wrench into shelf life protocols. Natural options are often less forgiving, so traditional, accelerated studies cannot be relied upon alone. Reformulation affects protocols for shelf life and stability testing, necessitating broader real-time and accelerated studies. Broader real-time testing becomes essential, factoring in variables like temperature fluctuations and packaging interactions. Laboratory involvement during the early stages of reformulation decreases the chances of failures or label discrepancies at later phases and ensures that labels match the ingredients in the product.
TABLE 1. Outbreak Characteristics Used for Hypothesis Generation (Credit: FDA)
“FDA has yet to establish a precise definition of 'natural,' making it difficult for manufacturers to meet regulatory demands while also satisfying consumer interpretations.”

Regulatory Landscape: What Should You Know?
FDA has yet to establish a precise definition of "natural," making it difficult for manufacturers to meet regulatory demands while also satisfying consumer interpretations. Flavors are regulated by 21 CFR 101.22 and are judged on whether they are sourced from natural sources without excessive processing,3 whereas color additives, whether natural or not, are subject to significantly tougher pre-market approval processes.
It is not just about government policies anymore, however. States are enacting their own prohibitions on synthetic colors, and popular chains such as Whole Foods and Walmart are establishing criteria that require transparent labeling, effectively rewriting the regulations as they go. California's synthetic dye ban, which goes into force in 2027, as well as analogous measures in New York and Illinois, require enhanced testing for trace synthetic substances. Retail manufacturers like Kroger now require third-party audits for natural claims, pushing laboratories to amplify documentation past Certificate of Analysis (COA) to include solvent usage logs and Generally Recognized As Safe (GRAS) attestation.
Laboratories need to be equipped for intense testing and piles of documentation to prove compliance. Then there is the GRAS provision, which determines whether something can honestly be called "natural." In this evolving environment, even a single extraction solvent, carrier, or incidental additive can decide whether an ingredient is taken into consideration as natural. The question becomes: how can companies protect themselves when the regulatory goalposts keep moving?
The 'Gray Zone' for Natural Flavors
Despite their popularity, "natural" claims are viewed by some consumers as a marketing strategy that leverages from the credibility of established organic regulations without actually adhering to "natural" standards. Incidental additives add another layer of complexity. In flavor extraction, solvents like hexane could disqualify as a "natural" label if residues exceed thresholds, according to FDA's incidental additives policy. Laboratories should track these complexities via targeted gas chromatography–mass spectrometry (GC–MS) protocols, preparing for audits that increasingly probe supply chain provenance.
The truth is that analytical chemistry alone cannot clear up this puzzle because many natural and synthetic flavor compounds are chemically identical, which makes traditional testing inadequate. While advanced techniques like Carbon-14 evaluation4 can help, they are pricey and infrequently utilized in recurring checks. As a result, manufacturers depend heavily on supplier transparency, robust COAs, and thorough audit trails.
This ambiguity suggests an important question: is "natural" something we can test for, or is it something we must verify through documentation and trust? FSQA teams depend heavily on documentation and certificates from flavor suppliers to validate a substance's "natural" status, rather than relying solely on analytical results. What if a supplier cuts corners? This is why building trust through audits and long-term contracts with suppliers is crucial.
“Validation of 'natural' claims is not optional; it is essential to shield against recalls and lawsuits.”

Maintaining the Stability of Natural Colors
Color is no longer a mere cosmetic variable—it is a strategic design facet that dominates product development, compliance, and brand equity. Manufacturers that act early will not only meet the new standards but also lead the new generation of clean-label food and beverage innovation. Natural pigments are generally more sensitive than their artificial counterparts and degrade over time due to oxidation and exposure to light. For products that depend on color elements for identification, getting rid of a visible cue can affect a consumer's trust in the product. An alteration in looks may result in confusion or reduced confidence in the product. Imagine a smoothie, originally beige in color, that turns to brown over time due to the effect of natural stabilizers. Even if the product continues to be safe, will consumers trust it?
The regulatory landscape is changing quickly, and consumers are becoming more conscious of how raw materials are sourced. Products with shorter ingredient lists are favored. Shoppers want more natural ingredients, but they also expect consistency of product. These trends put pressure on laboratories to predict volatility early, and they force product developers to reformulate without turning to artificial stabilizers. In many ways, natural colors compel organizations to revisit fundamental questions: what does quality look like, and how much variation will consumers accept?
Validating Natural Claims
When companies market their products as "all natural" or "100 percent natural," consumers have a right to believe them. Validation of "natural" claims is not optional; it is essential to shield against recalls and lawsuits. With evolving scrutiny, natural claims validation requires justifiable and meticulous documentation. Laboratories must design analytical approaches that focus on synthetic byproducts (such as certified dyes and specific solvents) and degradation products not found in natural substitutes. The validation should encompass the appropriateness of methods, low enough limits of detection, and regular verification testing consistent with product claims.
Enhancing laboratory data with comprehensive documentation from approved suppliers including COAs, in-depth process description, change controls, and audit reports will bolster the evidence necessary for inspection preparedness. Consumers demand the "right to know" where their food comes from, and producers must instill transparency through their supply chains to ensure safety, protect their brands, and verify product claims.
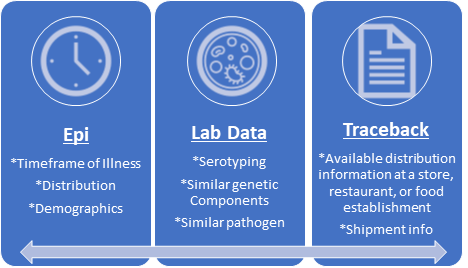
FIGURE 1. The Three Types of Evidence for Foodborne Illness Outbreak Investigations: Epidemiologic ("Epi"), Traceback, and Laboratory Data ("Lab Data") (Credit: FDA)
Beyond Compliance
According to food industry consultant Winston Boyd, "If one is conscientious about the meaning of 'all-natural,' the presence of these materials represents a problem. But it is entirely possible to find naturally derived alternatives to many synthetic emulsifiers."5 One of the biggest takeaways from the "natural claims" movement is that compliance is just the beginning. Companies going that extra mile do more than merely meet regulatory standards. They are proactively predicting ingredient shortages, diversifying their supply chains, investing in advanced technology, and building transparent communication with their consumers.
The shift to natural dyes and flavor additives is not a fleeting trend—it is a structural evolution of the food industry. Regulation, consumer behavior, and innovation are aligning in a single direction: cleaner, safer, and more transparent production systems.
References
- Shaak, E. "Fanta Berry Soda Mislabeled as Containing Only 'Natural Flavors,' Class Action Alleges." ClassAction.org. March 9, 2022. https://www.classaction.org/news/fanta-berry-soda-mislabeled-as-containing-only-natural-flavors-class-action-alleges.
- U.S. Food and Drug Administration (FDA). "HHS, FDA to Phase out Petroleum-Based Synthetic Dyes in Nation's Food Supply." April 22, 2025. https://www.fda.gov/news-events/press-announcements/hhs-fda-phase-out-petroleum-based-synthetic-dyes-nations-food-supply.
- Code of Federal Regulations. "Foods; labeling of spices, flavorings, colorings and chemical preservatives." 21 C.F.R. § 101.22. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-101/subpart-B/section-101.22.
- Gershon, H., A. Lykkeberg, F. Goren, and S. Mason. "Identifying Fraudulent Natural Products: A Perspective on the Application of Carbon-14 Analysis." Journal of Agricultural and Food Chemistry, 67, no. 49 (March 2019): 13393–13399. https://pubs.acs.org/doi/10.1021/acs.jafc.0c04391.
- Feder, D. "'All-Natural' a Legal Gray Area for Food and Beverage Labeling." Food Processing. October 29, 2009. https://www.foodprocessing.com/product-development/rd/article/11365764/ingredients-and-formulation-all-natural-a-legal-gray-area-for-food-and-beverage-labeling.
Shruti Nikam, M.S., PCQI, HACCP, SQF Advanced Practitioner works as an FSQA Manager at a baking ingredient company. She is a food safety and regulatory professional specializing in FSQA systems, labeling compliance, and quality management in manufacturing environments. She has extensive experience leading SQF audits, managing supplier approval programs, and supporting third-party certifications including Non-GMO, Halal, Kosher, and Organic. Shruti is a member of Phi Sigma Tau Honor Society and holds a master's degree in food safety and technology from the Illinois Institute of Technology.

