
SCROLL
DOWN
The journal Regulatory Toxicology and Pharmacology has retracted a paper from the year 2000 asserting the safety of glyphosate—the active ingredient in the herbicide Roundup—which has been relied upon since its publication as a hallmark piece of evidence for regulatory approvals of the agricultural chemical. In recent years, Roundup has faced increasing public scrutiny due to its potential adverse health effects like cancer, and Bayer's Monsanto, the maker of Roundup, has been mired in lawsuits alleging harm from exposure to the chemical. Bayer announced in 2021 that it would end sales of glyphosate-based herbicides in the U.S. to manage litigation risks, because, at the time, more than 40,000 related lawsuits had been filed by cancer patients or their families against the company.
According to the retraction, such lawsuits have uncovered emails from Monsanto showing that employees of the company may have contributed to the data collection, writing, and review of the 2000 study without acknowledgement. Further correspondence with Monsanto revealed during litigation also indicates that the authors may have received undisclosed financial compensation from Monsanto for their work.
The U.S. Environmental Protection Agency (EPA) is due to deliver a safety reevaluation decision for glyphosate in 2026 following a lawsuit filed by the Center for Food Safety (CFS) and its allies challenging the agency's determination that glyphosate is safe for humans and the environment. In 2022, the U.S. Court of Appeals for the Ninth Circuit overturned EPA's safety decision, finding that the agency did not adequately consider the chemical's risk of harm to humans or to endangered species in compliance with the Federal Insecticide, Fungicide, and Rodenticide Act (FIFRA). At the time, the court ordered the completion of a new assessment by October 2022, which EPA said it could not meet, forcing the agency to withdraw its previous decision. This required EPA to draft and issue a new, complete, FIFRA-compliant glyphosate final registration review decision, which is expected to be completed in 2026.

Journal Retracts Hallmark Glyphosate Safety Study, Increasing Cancer Concerns
Eat Real Food: New U.S. Dietary Guidelines Name and Shame 'Highly Processed Foods'
Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images

The U.S. Department of Health and Human Services (HHS) and the U.S. Department of Agriculture (USDA) have published the new Dietary Guidelines for Americans 2025–2030 (DGAs). Notable changes from the previous DGAs include an increased recommended daily protein intake, emphasis on the healthfulness of dairy, and sweeping advice to avoid "highly processed" foods as a category, as well as novel consideration of the gut microbiome.
Alongside the new DGAs is a reimagined, inverted food pyramid, at the top of which (the largest part) are "Protein, Dairy, and Healthy Fats," placed next to "Vegetables and Fruits"—both given equal importance—which narrow down to "Whole Grains" at the bottom (the smallest part). The new pyramid was released on realfood.gov, which states, "For decades we've been misled by guidance that prioritized highly processed food, and are now facing rates of unprecedented chronic disease… For the first time, we're calling out the dangers of highly processed foods," before introducing the new pyramid.
The advice for each person to adhere to a daily calorie budget that is reasonable for their physical characteristics and lifestyle (loosely around 2,000 calories per day) remains the same. Also unchanged is the general importance placed on whole grains (two to four servings per day), fruits and vegetables (five servings per day), and wholesome proteins, as well as caution against added sugars and alcohol. Some public health stakeholders have raised concerns about the DGAs' emphasis on animal protein, butter, and full-fat dairy, questioning the scientific basis behind these claims and whether they were influenced by industry interests.
The new DGAs explicitly state that "highly processed" foods such as "packaged, prepared, ready-to-eat (RTE), or other foods that are salty and sweet… that have added sugars and sodium" should be avoided. Instead, nutrient-dense, home-cooked foods should be prioritized. Also stated is advice to limit foods and beverages containing "artificial flavors, petroleum-based dyes, artificial preservatives, and low-calorie non-nutritive sweeteners," echoing HHS' and the U.S. Food and Drug Administration's (FDA's) general stance against food additives and chemicals under Secretary Kennedy, who is known for his "Make America Healthy Again" (MAHA) agenda.
The term "highly processed" as it is used in the new DGAs is reminiscent of the controversial and inconsistently defined term "ultra-processed foods" (UPFs). Swapping "UPFs" for "highly processed foods" in the new DGAs seems to circumvent ongoing debate about the definition of UPFs and whether it should be used for official health advice and rulemaking purposes. The term UPFs is typically associated with the four-category NOVA food classification system, which defines Category 4 UPFs as "industrially manufactured food products made up of several ingredients (formulations) including sugar, oils, fats, and salt and food substances of no or rare culinary use."
In the U.S., efforts at the federal and state levels to define UPFs and regulate the category are underway. Federally, FDA and USDA are considering public input on a uniform, recognized definition for UPFs. A joint request for information (RFI) closed in October, which received comments from diverse stakeholders with equally diverse opinions on how—or if—UPFs should be defined, and how the category should be regulated. Critics say the use of the term UPFs villainizes processing without nuance and the regulation of UPFs as a category could have unintended negative consequences.
A report published by the U.S. Government Accountability Office (GAO) on January 7, 2026 assessed FDA's ongoing implementation of the Food Safety Modernization Act (FSMA) and identified several areas requiring more work. In the 15 years since FSMA's passage, the agency has issued nine FSMA regulations that build a preventive foodborne illness framework, and completed most but not all requirements of FSMA, according to GAO.
The nine rules identified by GAO are:
- Preventive Controls for Human Food, updating FDA's Good Manufacturing Practices (GMPs) requirements and adding requirements for hazards analysis and risk-based preventive controls
- Preventive Controls for Animal Food, establishing GMP, hazards analysis, and risk-based preventive control requirements for animal food
- The Produce Safety Rule, establishing minimum standards for businesses that grow, harvest, pack, or hold certain fruits and vegetables, including agricultural water standards
- The Sanitary Transportation Rule, requiring sanitary transportation practices for those engaged in transporting food
- The Intentional Adulteration Rule, requiring facilities to address hazards that may be introduced intentionally to cause harm to public health
- Laboratory Accreditation for Analyses of Foods (LAAF), establishing a program to recognize bodies that will accredit laboratories to test food in certain circumstances
- The Food Traceability Rule (FSMA 204), establishing recordkeeping requirements for establishments that manufacture, process, pack, or hold foods included on FDA's Food Traceability List
- The Foreign Supplier Verification Programs for Food Importers (FSVP), requiring importers to verify that the food they import for humans and animals meets U.S. safety standards
- Accreditation of Third-Party Auditors, establishing a voluntary program for the accreditation of third-party certification bodies to conduct food safety audits of foreign entities and issue certifications.
GAO also identified 46 key FSMA requirements, of which FDA has fulfilled 41. While FDA has, for example, issued several implementation guidances for industry and conducted various studies required by FSMA, the agency has not:
- Issued final guidance on hazards analysis and preventive controls for human food, separate from animal food
- Finalized guidance to protect against the intentional adulteration, or tampering, of food
- Continued to report on the progress of implementing a national Food Emergency Response Laboratory Network (FERN), failing to do so in 2021 or 2023
- Published updated Good Agricultural Practices (GAPs) for fruits and vegetables in alignment with the Produce Safety Rule
- Implemented a system to improve FDA's capacity to track and trace food that is in the U.S. (FSMA 204).
GAO made several recommendations to complete FSMA requirements, with which HHS concurred:
- Establish a timeline for finalizing the guidance on hazards analysis and preventive controls for human food and issue the guidance
- Establish a timeline for finalizing the guidance to protect against the intentional adulteration of food and issue the guidance
- Publish a report in 2026 on the progress of implementing a FERN laboratory network and establish milestones and timelines for publishing future reports
- Establish milestones and timelines for updating the GAPs for fruits and vegetables as required by the Produce Safety Rule
- Establish an implementation plan for FSMA 204
- Develop and implement a performance management process to assess the results of FSMA regulations and their contribution to the prevention of foodborne illness.

GAO Identifies Areas in Which FDA Has Yet to Fulfill FSMA
The latest Cost Estimates of Foodborne Illness data published by USDA's Economic Research Service (USDA-ERS) suggests that the economic burden of foodborne illness in the U.S. for 2023 was $74.7 billion. USDA-ERS' estimates consider data on the costs of medical treatments, employment and wages, U.S. Centers for Disease Control and Prevention (CDC) data on the incidence of foodborne illness and associated hospitalizations and deaths, and peer-reviewed scientific research.
Of the $74.7 billion in estimated costs related to foodborne illnesses, the majority was attributed to serious illnesses caused by a specified pathogen. Although these illnesses represented only 20 percent of cases in 2023, they accounted for 60 percent of cost. Specifically, illnesses attributed to one of 31 major foodborne pathogens caused 9,388,133 cases costing $44,743,300,000 for a per-case cost of $4,766.
Conversely, milder foodborne illnesses for which the pathogen cause is not identified were responsible for 80 percent of cases and 40 percent of total cost in 2023. Specifically, unspecified foodborne gastroenteritis caused 38,392,704 cases costing $29,972,800,000 for a per-case cost of $781. The USDA-ERS estimates also broke down costs for important pathogens, which vary due to the extent and severity of illnesses they cause. Pathogen-specific total costs ranged from $100,000 for the very rare cholera to $17 billion for nontyphoidal Salmonella. Average per-case cost by pathogen ranged from $196 for Bacillus cereus to $4.6 million for Vibrio vulnificus.
USDA Estimates Foodborne Illnesses Cost U.S. $74.7 Billion in 2023
In December 2025, the Codex Committee on Food Hygiene (CCFH) decided to initiate work related to the control of Clostridium botulinum in powdered infant formula. The decision, prompted by the ongoing infant botulism outbreak linked to ByHeart-brand infant formula sold across the U.S., was made at the 55th Session of CCFH (CCFH55) in December 2025. Specifically, CCFH decided to request the Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Meetings on Microbiological Risk Assessment (JEMRA) to conduct a risk assessment on spore-forming pathogens, including C. botulinum and Bacillus cereus, in powdered infant formula.
Additionally, CCFH will ask JEMRA to 1) update the existing risk assessment and scientific advice on Cronobacter and Salmonella in powdered infant formula and 2) provide other relevant scientific advice that would inform recommendations on strengthened control measures across infant formula production, covering all stages from primary production and packaging through to the reconstitution of the product, and including environmental monitoring programs.
The decision was backed by the International Baby Food Action Network (IBFAN), which is calling for mandatory independent monitoring and accountability in national food regulatory systems and stricter manufacturing safeguards to mitigate the risk of infant exposure to C. botulinum spores from contaminated infant formula. IBFAN has raised concerns about gaps in the safety of powdered infant formula revealed by the ongoing ByHeart infant botulism outbreak, which, as of late December 2025, has affected 51 infants across 19 U.S. states. IBFAN asserts that Codex Alimentarius guidelines should be revised to reduce the food safety risk of C. botulinum in powdered infant formula manufacturing.
The group also asked that revisions to the Codex Guidance Code of Hygienic Practice for Powdered Formula for Infants and Young Children (CXC 66-2008) include warnings about the food safety risks of global online marketing and sales of powdered infant formula—another issue exemplified in the ByHeart botulism outbreak—forbidding any messaging that is not in compliance with WHO's International Code of Marketing of Breast Milk Substitutes.

Codex Initiates Work on Clostridium botulinum in Infant Formula
The European Commission has published a revised guidance document on monitoring and shelf life studies for Listeria monocytogenes in ready-to-eat (RTE) foods in compliance with Regulation (EU) 2073/2005 on the microbiological criteria for foods. The revised guidance reflects amendments made to the microbiological criteria for foods in November 2024, which expand the requirements for food business operators regarding L. monocytogenes and come into force July 1, 2026.
Originally, food business operators (FBOs) were only required to ensure that L. monocytogenes is undetectable in 25 grams (g) of RTE foods before the food left the immediate control of the manufacturing facility, in cases where the producer is unable to demonstrate that the level of L. monocytogenes will not exceed the limit of 100 colony forming units (CFU)/g throughout the shelf life of the foods concerned. Now, this responsibility is expanded to all situations where RTE foods that may support the growth of L. monocytogenes beyond 100 CFU/g are placed on the market throughout their shelf life.
The revised guidance document provides new information to FBOs about the evaluation of whether a RTE food supports the growth of L. monocytogenes, and if the new criteria apply to their situation. It also provides guidance on shelf life studies to demonstrate their foods comply with the criteria, including the selection, execution, validation, verification, and documentation of such studies. Information about environmental monitoring and food product testing are also included. The document may also assist competent authorities when performing official controls and third parties in the development of shelf life studies.

EU Provides Guidance on Shelf Life Studies to Reflect New Listeria Criteria for RTE Foods
The European Food Safety Authority (EFSA) has published guidelines for reporting whole genome sequencing (WGS) data to EFSA as required by Regulation (EU) 2025/179, defining acceptance criteria for WGS data quality, metadata standards, and procedures for voluntary and mandatory data submissions.
In February 2025, the EU adopted a new regulation requiring Member States to conduct WGS on the isolates of five important pathogens during the investigations of foodborne illness outbreaks and share relevant data with EU public health agencies. Specifically, Regulation (EU) 2025/179 states that, during official controls at food and feed businesses suspected of being implicated in a foodborne illness outbreak, competent authorities within Member States must collect isolates of Salmonella enterica, Listeria monocytogenes, Escherichia coli, Campylobacter jejuni, and Campylobacter coli derived from food, animal feed, and related environmental samples. Member States will be required to carry out WGS on those isolates. WGS results must be transmitted to EFSA, which developed a joint One Health system together with the European Center for Disease Prevention and Control (ECDC). Within the joint One Health system, EFSA will be able to compare WGS data for food-related isolates with WGS data from human isolates that are communicated to ECDC.
The new guidelines, published on December 12, 2025, focus on the critical requirements and submission procedures that reporting countries must follow to ensure compliance with regulatory obligations under Regulation (EU) 2025/179 and EFSA criteria when submitting WGS data to EFSA. The guidelines describe the scope of the data collection, which includes raw sequencing reads, genome assemblies, and typing results, along with mandatory metadata such as epidemiological and outbreak investigation data for submissions linked to foodborne illness outbreaks.
Additionally, roles and responsibilities are clearly defined in the guidelines: country officers coordinate submissions at the national level, reporting countries manage data release, and submitters handle technical uploads. The document also addresses the data ownership, intellectual property, and the use of human-origin data in compliance with EU privacy regulations.

EFSA Publishes Guidelines for Mandatory WGS Data Submission During Outbreaks
The European Commission has announced that it will significantly increase and strengthen its controls for animal, plant, and food imports entering the EU, with the intent of improving food safety for consumers and leveling competition for European agri-food producers.
The new measures to be implemented at EU borders and in third countries include:
- A 50 percent increase in the number of audits carried out on non-EU countries over the next two years, while maintaining the level of control in EU countries
- A 33 percent increase in audits of European Border Control Posts to verify that Member States are carrying out border inspections in line with EU requirements
- Closer monitoring of non-compliant commodities and countries, with the frequency of checks increasing as necessary
- Commission support to Member States in carrying out additional checks
- Establishment of a dedicated EU Task Force to be launched in early 2026, which will make import controls more efficient and focus especially on pesticide residues, food and feed safety, and animal welfare, as well as consider coordinated EU monitoring action on specific imported products
- Training for approximately 500 national authority staff on official controls through a dedicated EU program
- Updated rules on allowing the import of products with traces of particularly hazardous pesticides that are banned in the EU, harmonizing with recently updated international standards.
These measures align with the EU's Vision for Agriculture and Food, unveiled in February 2025, of which a "non-negotiable element" is improving imported food and feed safety.

EU to Increase Audits of Agri-Food Importers by 50 Percent Over Next Two Years
A recent study underscores the complexities of using novel data streams (NDSs)—such as crowdsourced reports and social media posts—to detect foodborne illness outbreaks. While these technologies can expedite identification compared to traditional surveillance, they also carry risks of amplifying false signals before confirmation by public health authorities.
For the study, researchers surveyed Minnesotans using a discrete choice experiment to determine which headline attributes influence individuals to self-identify as ill during a publicized foodborne illness event. The experiment tested four factors: the number of people reported sick, symptom descriptions, FDA involvement, and a call to action.
Results showed that all four attributes increased the likelihood of self-identification, with the strongest drivers being the number of reported illnesses and inclusion of symptoms such as nausea, vomiting, and diarrhea. Specifically, the odds of self-identifying as a victim of a foodborne illness outbreak more than doubled when the number of people publicized as ill reached 8,500 or when symptoms were reported.
Based on their findings, the researchers underscore that publicizing unconfirmed signals of foodborne illness can lead to misattribution and overreporting, driven by psychological and social factors—a critical limitation of NDS-based outbreak detection. A notable example of this phenomenon occurred in 2022 when a crowdsourced platform flagged dry breakfast cereal as a potential source of illness, prompting media coverage and thousands of self-reports, despite subsequent investigations finding no link.
Although the study highlights limitations of NDSs and their ability to amplify false signals, it provides valuable information about which attributes people respond to in a publicized outbreak headline. Future research focused on message development could help public health authorities develop messages that maximize the likelihood of garnering responses to a call to action (e.g., reporting illnesses to the appropriate health agency).
Overall, the researchers advocate for reporting systems managed by regulatory agencies including rapid confirmation protocols to prevent premature announcements that could harm consumer trust and industry reputation. They emphasize that, as digital tools evolve, balancing timeliness with accuracy will be essential for effective outbreak management and food safety protection.

Study Investigates Influence of Social Media on Foodborne Illness Outbreak Identification

Brendan Niemira, Ph.D. joined the Institute of Food Technologists (IFT) as Chief Science and Technology Officer. Dr. Niemira formerly served as a lead scientist and researcher at USDA's Agricultural Research Service for more than 25 years.

NIEMIRA
The UK Food Standards Agency (FSA) appointed Ian Young, M.D. as its new Chief Scientific Adviser.
Kelly Leighton was named the new Executive Director of the Center for Food Integrity (CFI).

LEIGHTON
The Refrigerating Engineers and Technicians Association and the Refrigeration Service Engineers Society (RETA-RSES) appointed Vern M. Sanderson as the Association's new Executive Director.
Mindy Brashears, Ph.D. was confirmed by the U.S. Senate for a second term as USDA's Under Secretary of Agriculture for Food Safety. She previously served in the same position from 2020–2021 during President Trump's first term.

BRASHEARS
The GS1 US Board of Governors has elected Smita Katakwar, Senior Vice President of Technology at Wegmans Food Markets, Inc., to help guide the GS1 US strategy toward greater cross-industry adoption and use of GS1 Standards for increased supply chain efficiency and visibility.
Jim Mazany was appointed as P.F. Chang's new CEO.

MAZANY
Kraft Heinz named former Kellanova CEO Steve Cahillane as CEO as it prepares to split into two companies. Carlos Abrams-Rivera will step down as CEO and serve as an advisor.
Sprague Pest Solutions promoted Paul Treleven to Vice President of Sales.

TRELEVEN
AeriTek has expanded its foodservice leadership team with the appointments of Sean McGrann as Vice President of Sales, Foodservice North America, and Anthony Tortoriello as Director of Sales, Foodservice U.S. and Canada.
Chris Caldwell was promoted to National Sales Manager of Robroy Industries.
Food Safety Magazine is saddened to announce the passing of Dr. Frank Busta on January 24, 2026. An internationally recognized food safety expert, Dr. Busta received the magazine's Distinguished Service Award in 2021 for his outstanding service to food safety science. Read more about his contributions to food safety here.

EPA Approves First Protein-Based Biofungicide
EVOCA, a new biofungicide from Biotalys, has received regulatory approval from the U.S. Environmental Protection Agency (EPA). The plant protection product was developed using Biotalys' AGROBODY technology platform and is the first protein-based biofungicide of its kind to be approved by EPA. At the end of October 2025, EPA also posted a final rule exempting EVOCA's active ingredient residues on treated crops from tolerance requirements. No maximum residue limits will apply given EVOCA's safety profile.
EVOCA is a precision biocontrol solution with a new mode of action, recognized by the Fungicide Resistance Action Committee, that targets the fungal pathogens botrytis (grey mold) and powdery mildew in high-value fruits and vegetables while minimizing the risk to beneficial organisms or the environment. Biotalys positions EVOCA as a novel, sustainable solution for growers to protect their crops, with the flexibility for use in both pre- and post-harvest applications across a wide range of food crops in both greenhouse and outdoor growing conditions.
With EPA's approval, Biotalys will proceed with the dossiers for state registrations in California and Florida. In Europe, EVOCA has entered the peer review phase. The Netherlands has proposed its approval in Europe, subject to the submission of certain additional data as requested during the peer review phase.

Five More Companies Join Alliance to Stop Foodborne Illness
The Alliance to Stop Foodborne Illness has announced new industry members: Amazon, Amerisan, The Meat Institute, Mérieux NutriSciences, and Yum! Brands. The Alliance is a collaborative effort to advance organizational food safety culture, thereby preventing foodborne illness and protect consumers. Since its launch in 2018, the Alliance to Stop Foodborne Illness has grown from ten founding companies to 25 industry partners. The Alliance's unique model brings together industry leaders, consumers, academics, and regulatory stakeholders who recognize that food safety is a shared responsibility.

Brazil Approves MFGM Product as Food Ingredient

Arla Foods Ingredients' Lacprodan MFGM-10 has been approved by the Brazil Ministry of Agriculture, Livestock, and Food Supply (MAPA) for use in food and beverages in which whey protein concentrate is permitted. Lacprodan MFGM-10 is part of Arla Foods Ingredients' range of milk fat globule membrane (MFGM) products, which contain whey protein as well as complex milk lipids and other nutrients.
Occurring naturally in breast milk, MFGM is best known as an infant formula ingredient, but also offers benefits for adults and older children. The decision in Brazil follows two other regulatory developments in 2025. In April, it was confirmed that MFGM is not classified as a novel food in the EU, allowing it to be declared on products for both infants and adults. In July, MFGM was approved for use in infant formula products in Australia.
CAG, FSLG Partner to Offer Food Safety Culture Solutions Across Borders

Culture Advisory Group Inc. (CAG)—a Canadian food safety governance, quality, and risk management solutions provider—is expanding into the U.S. through a strategic partnership with the Food Safety Leadership Group (FSLG). The partnership unites two complementary organizations to provide food, beverage, and supplement companies across North America with an approach to food safety that goes beyond compliance to enterprise-wide risk optimization.
Developed through years of working with Canadian and global food companies, CAG offers administrative and governance frameworks, proprietary risk management software, and board-level advisory services. FSLG contributes industry technical expertise, senior-level operational field experience, and established relationships with U.S. food manufacturers. Together, the two companies leverage a full spectrum of capabilities that enable them to help companies build a food safety culture, from the production floor to the boardroom.
Operating under the CAG banner, the partnership provides multinational companies and cross-border operations with a unified oversight of their top food safety and quality risks. Instead of navigating different approaches or providers in each country, CAG clients gain consistent methodology, integrated reporting, and coordinated support across their entire North American footprint.
Researchers Develop Active Packaging That Senses, Preserves Food Quality
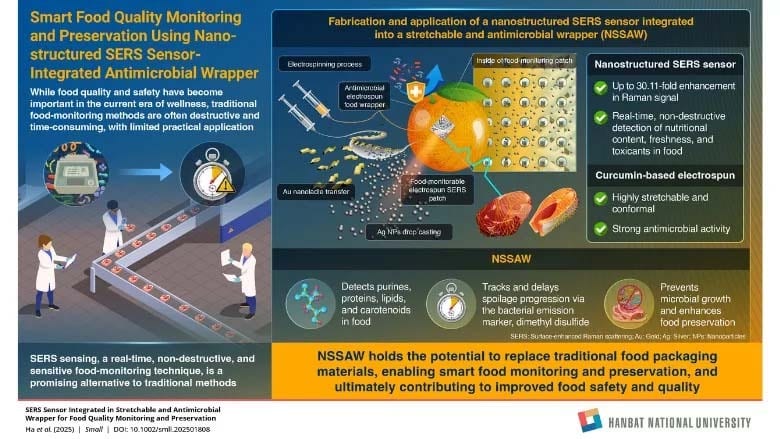
Researchers from Hanbat National University, Korea University, and Korea Institute of Machinery and Materials have developed a surface-enhanced Raman scattering sensor integrated into stretchable and antimicrobial wrap for real-time food quality monitoring and preservation. The two-in-one nanostructured SERS sensor is integrated into a stretchable, antimicrobial wrapper (NSSAW) that monitors and actively preserves food quality. The wrapper incorporates a nanostructured SERS sensor that delivers up to 30.11-fold Raman enhancement, enabling real-time, non-destructive detection of nutritional components. At the same time, the curcumin-thermoplastic polyurethane (TPU) electro-spun wrapper shows strong antimicrobial efficacy of 99.99 percent against Staphylococcus aureus and 99.9 percent against Escherichia coli, helping extend shelf life.
In cold chain logistics and storage, the wrapper could help distributors decide when to ship and sell food by continuously tracking freshness and spoilage chemistry. In retail smart packaging, its stretchable, conformal, and biocompatible nature enables non-destructive, on-package checks of quality and nutrition markers—without causing damage to food—supporting point-of-sale quality automation and transparent date labeling. NSSAW could also act as an on-food freshness indicator during consumer storage for home use and meal kit delivery, linking chemical changes to easy-to-interpret signals over time. In addition, for high-value seafood and meats, quantitative tracking of purines such as hypoxanthine supports premium-grade verification and shelf-life decisions.
Global Research Report Reveals Gaps, Opportunities in Auditing

A global research report published by World of Auditing reveals that food safety auditing is at a pivotal turning point, outlining longstanding challenges and significant opportunities. The report is based on extensive global dialogue and a survey of auditors and industry professionals, providing data-driven insights for auditing modernization to ensure it remains effective, trustworthy, and equipped for future challenges.
Despite its central role in consumer protection, food safety auditing has lacked the systematic research seen in fields like financial or social auditing. Insights have long been scattered across standards, practices, and individual experiences. Addressing this gap, experts from World of Auditing accelerated momentum for change beginning with an article published in the February/March 2023 issue of Food Safety Magazine, titled, "Why a Paradigm Shift Is Needed in Food Safety Auditing." The article sparked global debate, a think tank, a widely circulated whitepaper, and conversations held at conferences around the world.
The newly published report answers the call for clarity about the state of food safety auditing. The findings point to a system that is under pressure, yet full of potential through empowered internal audits, stronger auditor development, risk-based approaches, and digital innovation. The report is available in two formats—a Core Report and an Extended Report—both of which can be accessed on World of Auditing's website.
RESOURCES
The World Health Organization (WHO) has released a set of updated manuals to help national authorities strengthen foodborne illness outbreak surveillance and response, contributing to faster and more reliable communication through the International Food Safety Authorities Network (INFOSAN). Notifications shared through INFOSAN are first detected at the national level, and the speed and clarity of early information influence subsequent decisions and actions. The updated manuals explain how countries can strengthen these systems so that signals of concern are identified sooner, verified reliably, and shared through INFOSAN when there are international implications.
The manuals describe how to integrate laboratory data, environmental assessments, food chain information, and public health investigations to support more comprehensive risk assessments and strengthen the evidence supporting notifications sent to INFOSAN. Additionally, they reflect emerging priorities, including the growing impact of climate and environmental change on foodborne risks and the expanding role of integrated surveillance across the food chain.
WHO Publishes Manuals for Strengthening National Foodborne Illness Surveillance and Response Systems

The European Commission recently published a dashboard that maps all food fraud cases covered in its monthly food fraud reports since 2016. At present, it includes more than 2,000 recorded cases, and it will be continuously updated with new cases. The application allows incidents to be filtered by commodity, region, fraud type, year, and other qualities. It bolsters food defense efforts by enabling the identification of trends, supporting risk assessment, and informing decision-making.
EU Publishes Food Fraud Tool
Food Standards Australia New Zealand (FSANZ) has published a new Work Program Dashboard, which provides a high-level overview of FSANZ's current work and priorities, including food applications, standards development, food safety and surveillance, international engagement, and other areas. The dashboard, which will be updated regularly, highlights key projects, regulatory reviews, and other FSANZ initiatives that contribute to ensuring safe and suitable food, a healthy food supply, informed consumers, and strong food economies.
FSANZ Releases Dashboard of Current Food Safety Work
The European Commission has published a document to answer frequently asked questions about the requirements of new EU legislation that mandates whole genome sequencing (WGS) testing and data reporting for important foodborne pathogens. Regulation (EU) 2025/179 comes into force on August 23, 2026.
Commission Implementing Regulation (EU) 2025/179 aims to facilitate the swift identification of causes of a foodborne illness outbreak and the related batches, lots, or consignments of potentially unsafe food by requiring WGS analysis and reporting to the European Food Safety Authority (EFSA) for isolates of Salmonella enterica, Campylobacter jejuni and Campylobacter coli, Listeria monocytogenes, and Escherichia coli. When one of these pathogens are suspected to be associated with a foodborne illness outbreak, at least one isolate obtained from animals, feed, food, or the feed/food production environment must be analyzed and data must be submitted to EFSA.
In the case of a multinational outbreak, the competent authority of each Member State where an isolate was detected, and where the isolate is associated or suspected to be associated with an outbreak, are responsible for carrying out WGS. Food businesses must submit isolates to competent authorities for WGS, upon request. The data to be submitted to EFSA along with WGS sequences include reference numbers, pathogen species, and date and Member State of samplings, as well as the description of the food, animal species, feed, or environment from which the isolate was derived. Additionally, laboratories conducting WGS analyses should be ISO 17025-accredited.
The European Commission's Q&A document can be found here.
European Commission Publishes Q&A Document for Mandatory WGS Testing and Reporting


