REGULATORY REPORT
By Michael C. Bazaco, Ph.D., Stelios Viazis, Ph.D., Don Obenhuber, Ph.D., Patricia Homola, Ph.D., Fazila Shakir, M.H.S., and Angela Fields, M.P.H.
An Overview of Historic Foodborne Illness Outbreak Investigations Linked to the Consumption of Sprouts: 2012–2020
Sprout production is inherently conducive to the survival and growth of pathogens

Photo credit: karma_pema/E+ via Getty Images
SCROLL DOWN
Sprouts are commonly consumed raw and are used in a variety of ways in culinary dishes. However, between January 1996 and August 2018, the U.S. Food and Drug Administration (FDA) reported 50 outbreaks linked to contaminated sprouts, which resulted in more than 2,600 cases of foodborne illness.1 Generally, production of sprouts may include some of the following steps: seed receipt, seed storage, initial seed rinse, seed treatment, pregermination seed soak, germination and growth, microbial testing of spent sprout irrigation water (or in-process sprouts), harvest, wash and drain sprouts, bulk cool or spin dry, pack and/or package, cooling and storage, and finally, distribution.2 There are specific food safety issues associated with sprouts due to the conditions under which they are produced. The typical time, temperature, water activity, pH, and available nutrients during production are ideal for the survival and growth of pathogens, if present.
We present here a brief overview of historic investigation information associated with outbreaks linked to sprout consumption between 2012 and 2020 that were investigated by FDA’s Coordinated Outbreak Response and Evaluation (CORE) Network.3 In addition, we outline the efforts to identify and respond to these outbreaks, the protective regulatory and public health actions taken to respond to them and prevent future outbreaks, investigational challenges encountered, possible contamination routes, and how the Food Safety Modernization Act (FSMA) Produce Safety rule (PSR) and the Food Traceability proposed rule may affect sprout safety.3–5
Responding to Outbreaks Linked to Sprouts and Associated Challenges
During any given outbreak investigation, FDA and the U.S. Centers for Disease Control and Prevention (CDC), along with state and local authorities, collect and analyze three types of evidence: epidemiologic information, traceback data, and laboratory analyses of food and/or environmental samples taken from food production environments and retail outlets. Because each outbreak is unique and the information available to investigators varies from outbreak to outbreak, each individual type of information impacts the collection and analysis of the other two. Through rigorous analysis of the information collected, investigators often can identify a likely or confirmed food source of an outbreak. Investigations of foodborne illness outbreaks linked to sprouts employ these same methods but also encounter some unique challenges. In addition to the usual epidemiologic challenges such as collecting complete and accurate food histories from cases, epidemiologic investigations of sprouts are difficult because people have trouble remembering what type of sprouts they may have consumed and/or where they consumed them. In some investigations, sprouts can be a hidden ingredient in prepared meals such as salads, where the person may not even know that they consumed them.
Traceback investigations can be difficult to conduct on sprouts for a number of reasons. These include issues with determining the point of contamination (e.g., during seed production or storage, and/or where the seeds are sprouted, during transportation or at retail) and the difficulty with collecting records. Traceback investigations can be challenging and limited when there is a lack of supply chain documentation as a result of poor record keeping. For sprouts, a lack of seed lot traceability due to commingling of seed lots throughout production, particularly at the distributor/supplier level, is also a common challenge. Furthermore, in an ideal traceback investigation, convergence on one source of sprouts or seeds will occur. However, even when only a single sprout grower is identified through traceback, the source of sprout contamination can be difficult to determine as contamination could have originated earlier in the supply chain on the seeds used for sprouting, during seed conditioning or cleaning, or from the conditions in that particular sprouting operation. The ideal conditions to grow sprouts (temperature, humidity, wet environment, etc.) can also lead to the growth and proliferation of pathogenic microorganisms, and therefore even a low level of contamination on seed can cause widespread contamination events. In the case of convergence of the traceback at one sprouting operation, it takes additional investigational work to determine whether the contamination was at the seed level, such as sampling and testing the seed itself. On the other hand, if multiple sprout growers are identified through traceback, investigators can more readily determine whether the contamination was introduced by seeds.
FDA or state investigators collect product suspected of causing illness for analysis as well as samples of the production environments in which the sprouts are produced. During an outbreak investigation, the laboratory analysis of food and/or environmental samples taken from food production operations is typically conducted by state or FDA laboratories. These may include both sprouts and seeds, samples of spent sprout irrigation water from the sprouting operation, and/or samples taken from surfaces in the production environment. Molecular subtyping can link the DNA from a strain of a bacterial pathogen from sprouts or from a sprout operation with a clinical sample from a patient who has become ill. A common challenge in sampling sprouts is that contamination is typically not present homogenously throughout a specific production lot or shipment of sprouts or seeds. An additional challenge is that although laboratory analysis of sprout or seed samples can confirm the presence of contamination, it cannot determine where in the supply chain the contamination occurred (e.g., during seed production and processing, during seed shipment, or as a result of environmental contamination during sprout production). Finally, sampling of both the products and spent sprout irrigation water is limited to what is available at the time of sampling, which may not be the same product associated with the outbreak; however, it could help determine whether there is an ongoing contamination issue at the facility that is a continued risk to public health.
FIGURE 1. Number of illnesses with the outbreak strain for each outbreak (n = 14) of foodborne illnesses linked to the consumption of sprouts, by month of illness onset in the United States, 2012–2020.
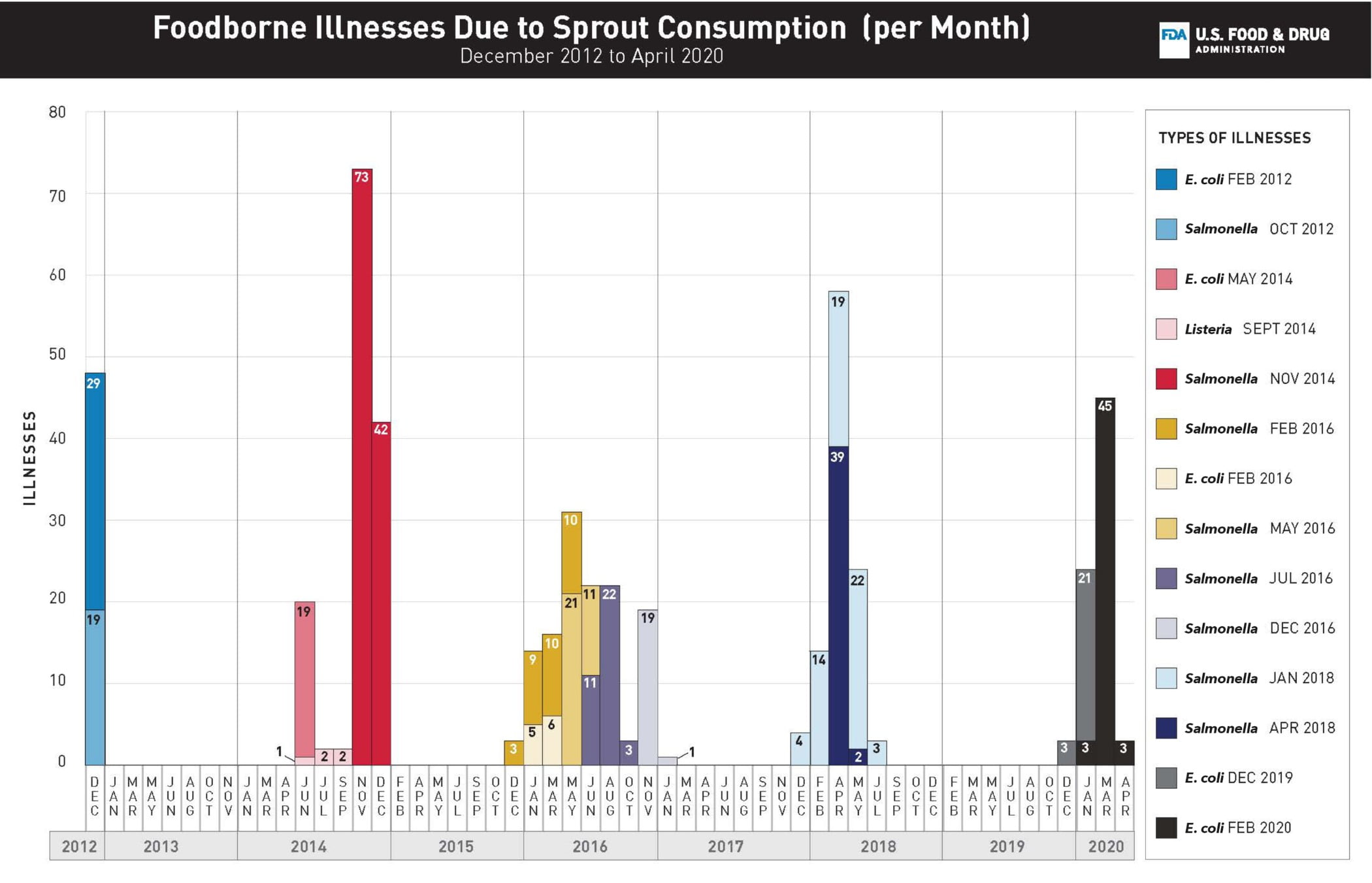
Outbreak case distribution data collected from the CDC Foodborne Disease Outbreak Surveillance System for outbreaks between 2012 and 2018 and from public CDC and FDA web postings for outbreaks in 2019 and 2020.
Overview of Foodborne Illness Outbreaks Related to Sprouts
Between 2012 and early 2020, the FDA’s CORE Network, in collaboration with CDC, and state and local public health and regulatory partners, coordinated the response efforts for 14 multistate, sprout-related outbreak investigations involving 496 laboratory-confirmed human illnesses, 82 hospitalizations, and three deaths across 45 states (Figure 1). While this is not a comprehensive list of all outbreaks linked to sprouts over the period, it does include all outbreaks investigated by FDA. The consumption of sprouts was likely linked to outbreaks of Salmonella spp.,6 Shiga toxin-producing Escherichia coli (STEC),4 and Listeria monocytogenes7 infections (Table 1).
Investigators routinely conduct traceback investigations by reviewing supply chain records to identify the origin of food served or sold at a specific point of sale. Investigators confirmed that clover (4 of 14), mung bean (2 of 14), or alfalfa (5 of 14) sprouts were responsible for 11 of the 14 outbreaks, while the specific type of sprout could not be determined for one outbreak but was either clover, mung bean, or alfalfa sprouts. Mung bean sprouts were suspected but not confirmed for the two remaining outbreaks. Traceback investigations allow investigators to identify a common point shared by multiple points of sale, such as shared facilities, land, water sources, geographic regions, and others. Traceback investigations were conducted for all 14 outbreaks in this report, with nine investigations resulting in convergence at the seed supplier or sprouting level, while the remainder did not. Out of the outbreak investigations where there was convergence, six of the nine (67%) traceback investigations converged at the seed distributor/supplier, while the remaining three (33%) outbreaks converged at a sprout grower. The traceback investigations identified that two of these three sprout growers each sourced their seeds from a single supplier, while one of the three sourced their seed from multiple suppliers from multiple countries. During one traceback investigation, the outbreak strain was identified at one seed distributor/supplier through laboratory testing of seed samples. One traceback investigation identified a seed grower, and samples of seed from that grower confirmed the presence of the outbreak strain. For the traceback investigations that did not result in convergence, many factors contributed to the inability to draw conclusions. Of the 14 traceback investigations conducted, investigators determined that seeds were from both imported and domestic sources. In many investigations, there was a lack of traceability information to enable FDA to identify specific lots used for the distribution of the product in the outbreaks. Additionally, in several traceback investigations, there was a lack of available records to identify the entirety of the seed’s supply chain or to determine a common point where contamination of the seed could have occurred. In addition to issues with record collection and linking shipments across the supply chain domestically and internationally, information sharing can be impacted by lack of confidentiality agreements with some of the countries identified by the traceback investigations.
TABLE 1. Summary of foodborne illness outbreaks linked to the consumption of sprouts from 2012 to 2020.
“Investigations of foodborne illness outbreaks linked to sprouts…encounter some unique challenges.”


Sprout Outbreaks and Sources of Contamination
Based on the epidemiologic information available and the traceback data that led to convergence at the seed distributor or supplier, FDA concluded contaminated seeds were the most likely source of contamination in most of these outbreaks. Despite seed for sprouting being considered “food” under the Federal Food, Drug, and Cosmetic Act of 1938, the end use of seed may be unknown by those who grow the seed or by the conditioners and distributors who handle it, which may result in these entities’ not dedicating staff and resources to ensuring food safety best practices. FDA has observed conditions that can contribute to contamination at operations that grow and condition seed for sprouting, including farms that allow animals to graze on the growing seed crops, inadequate cleaning practices, and the presence of birds and rodents in and around seed conditioning areas and equipment.
Seeds may become contaminated at any point in the supply chain up to and including the sprout grower (e.g., at the seed grower, conditioner, during transport, or at the seed supplier). Past outbreak investigations have shown that poor practices at individual sprout growers may have contributed to the production of contaminated sprouts that led to illnesses, but contamination originating at a single sprout grower does not account for all the illnesses reported. Although the outbreak strain was identified in a seed sample during two of the outbreak investigations noted in this paper, past research has indicated that seed contamination may be sporadic and occur at very low levels8 and as a result can be difficult to detect. Laboratory analyses have frequently been unable to isolate pathogens from lots of seed that have been conclusively implicated in outbreaks of foodborne illness.9
During outbreaks that converged at a sprout grower, or where no point of convergence was identified, the source of contamination was less clear. It is possible that the use of contaminated seed contributed to these outbreaks, along with poor food safety practices by the sprout grower or others within the supply chain.7 Although this overview could not definitively determine at what point in the supply chain the seed was contaminated, the epidemiologic and laboratory evidence suggest that contaminated seed is a likely cause of most sprout outbreaks based on the data from 2012 to 2020.
FDA Response to Outbreaks
In an effort to protect public health and in response to any given outbreak, FDA uses the available investigational evidence to pursue several different responses. For example, if an importer of seeds implicated in an outbreak is identified, FDA is able to increase import screening with the goal of preventing additional contaminated product from entering the market. Once the source of the contaminated sprouts and/or seeds is identified, they can be recalled from the market if possibly contaminated or contaminated product is still there. During outbreaks where contaminated seed lots are identified, the producers are advised to have them destroyed, reconditioned, or discontinue their use for human food. FDA has issued warning letters to seed and sprout producers and to retailers who sell contaminated sprouts.
FDA also entered into the first consent decree of permanent injunction against a sprout grower for violating standards under the Produce Safety rule. The consent decree prohibits the defendants from growing, harvesting, packing, and holding sprouts at or from their operation, or any other operation, until certain requirements are met.6 It requires the defendants to, among other things, take corrective actions and notify FDA before such operations may resume.
“A common challenge in sampling sprouts during outbreaks is that contamination is typically not present homogenously throughout a specific production lot or shipment of sprouts or seeds.”


The FSMA PSR, Proposed Food Traceability Rule, and Sprouts
Like any produce that is consumed raw, sprouts served on salads, in wraps, in sandwiches, or other foods may contain bacteria that can cause foodborne illness. Sprouts present a special concern for contamination with human pathogens compared with other produce because the warm and humid conditions seeds and beans need to sprout and grow are also ideal for the growth of pathogenic bacteria. Pathogens present on seed may be able to survive for several years, and any bacteria present can multiply dramatically during the sprouting process. Organic or locally grown sprouts are not necessarily less risky and neither are sprouts grown at home. Washing sprouts may reduce risk but will not eliminate it.
The PSR requires, among other things, that covered sprout operations take measures reasonably necessary to prevent the introduction of known or reasonably foreseeable hazards into or onto seeds or beans used for sprouting [21 C.F.R. 112.142(a)] and use seeds that have been treated as described in the PSR [21 C.F.R. 112.142(e)].5 Furthermore, they are required to test spent sprout irrigation water (or, in some cases, in-process sprouts) for the presence of certain pathogens (21 C.F.R. 112.147). In addition, sprout operations are required to test the growing, harvesting, packing, and holding environment for the presence of L. monocytogenes and take corrective actions when needed (21 C.F.R. 112.145). Furthermore, the PSR requires that the sprouting operation take certain corrective actions if they know or have reason to believe a lot of seeds is contaminated with a pathogen [21 C.F.R. 112.142(b)]. For example, if the seeds or beans are associated with an outbreak of foodborne illness, the sprout operation must discontinue use of all seeds or beans from that lot, ensure that sprouts grown from that lot of seeds or beans do not enter commerce, and report the information to the seed grower, distributor, supplier, or other entity from whom the operation received the seeds or beans [21 C.F.R. 112.142(b)].
Section 204(d)(1) of FSMA requires FDA to undertake rulemaking to establish additional record-keeping requirements (beyond what is required in existing regulations) for facilities that manufacture, process, pack, or hold foods that FDA designates as high-risk foods.10 To further this objective, FDA published the Requirements for Additional Traceability Records for Certain Foods proposed rule (Food Traceability proposed rule) on September 23, 2020. The proposed requirements are meant to facilitate rapid and effective tracking and tracing of the designated foods to prevent or mitigate foodborne illness outbreaks.10 The proposed food traceability list was developed to establish which foods would require additional traceability records.11 As noted in the proposed rule, the additional record-keeping requirements would also apply to foods that contain those foods on the list as ingredients. The current draft list includes, among other foods, all varieties of fresh sprouts. The proposed rule, if finalized, would therefore assist in facilitating more rapid and effective traceback investigations linked to consumption of sprouts.
Conclusions
Foodborne illness outbreaks linked to the consumption of sprouts have been particularly challenging for investigators through the years, particularly with respect to the traceback to assist in identification of the outbreak source. These challenges could be addressed in part through improved traceability in the seed-for-sprouting supply chain, as well as better transparency throughout the industry regarding the conditions under which seed is grown and produced. As these investigations show, contaminated seeds are the likely cause for most sprout outbreaks, and FDA has released a draft guidance for industry titled Reducing Microbial Food Safety Hazards in the Production of Seed for Sprouting.12 It is important to always consider that foodborne pathogens can contaminate, survive, and grow under a variety of conditions, but even more so in the conditions established for the production of sprouts. However, as consumers continue to use sprouts in a variety of ways in culinary dishes, sprout operations must follow the applicable requirements in the PSR to protect public health.
The responses to these outbreaks included numerous public health officials at local and state health departments and public health laboratories in the U.S., who serve as the backbone of any multistate outbreak investigation. The assistance of state partners, including Rapid Response Teams, is crucial in the collection and analysis of product samples, traceback documents, and epidemiologic information. Special thanks to FDA’s emergency response coordinators, the Office of Regulatory Affairs’ Division of Import Operations, the Office of Compliance, as well as all other FDA staff that are instrumental in the outbreak response and coordination for their tireless efforts and assistance.
References
https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-final-rule-produce-safety.
Ferguson, D.D., et al. 2005. “Temporally Distinct Escherichia coli O157 Outbreaks Associated with Alfalfa Sprouts Linked to a Common Seed Source—Colorado and Minnesota, 2003.” Epidemiol Infect 133: 439–447.
National Advisory Committee on Microbial Criteria for Foods. 1999. “Microbiological Safety Evaluations and Recommendations on Sprouted Seeds. National Advisory Committee on Microbiological Criteria for Foods.” Int J Food Microbiol 52: 123–153.
Fett, W.F., T.‐J. Fu, and M.L. Tortorello, “Seed Sprouts: The State of Microbiological Safety,” in Microbiology of Fresh Produce, ed. Karl R. Matthews (Washington, DC: ASM Press, 2005), 167–219.
https://www.fda.gov/food/food-safety-modernization-act-fsma/fsma-proposed-rule-food-traceability.
https://www.fda.gov/food/food-safety-modernization-act-fsma/food-traceability-list#List.
Michael C. Bazaco, Ph.D., is a member of FDA’s CORE Network, which manages surveillance, response, and postresponse activities related to illness outbreaks in FDA-regulated human food and cosmetic products. Prior to coming to CORE, he was a member of the FDA Office of Analytics and Outreach, where he was the emerging infectious disease lead.
Stelios Viazis, Ph.D., is a member of FDA’s CORE Network and part of the CORE Outbreak Analytics team. Previously, he served as a member of CORE Response, where he coordinated outbreak response efforts as well as the FDA Produce Safety Network, where he served as a Western Region subject-matter expert on the Produce Safety rule.
Don Obenhuber, Ph.D., is a member of FDA’s CORE Network and as part of the CORE Outbreak Evaluation team, he is responsible for outbreak data analysis and trending, recommendations for improvement, and developing historical evaluation reports. In his 4-year tenure at CORE, he has managed the post- response activities of 42 outbreaks. Prior to joining CORE, he was an FDA drug reviewer for the Biologics and Manufacturing Branch, Center for Drug Evaluation and Research, performing in-depth scientific review and evaluation of microbiology data for therapeutic proteins and conducting preapproval inspections of establishments manufacturing biological therapeutic products.
Patricia Homola, Ph.D., is a consumer safety officer in the Division of Produce Safety at FDA's Center for Food Safety and Applied Nutrition (CFSAN). In this role, she provides subject-matter expertise on food safety issues related to sprouts and seed for sprouting.
Fazila Shakir, M.H.S., is a senior regulatory health adviser in the Office of Food Safety/Division of Produce Safety at CFSAN. In this role, she has led the development of policies, regulations, and guidance related to the safety of fresh produce, including sprouts, under FSMA.
Angela Fields, M.P.H., is the senior consumer safety officer for the CORE Network. Her responsibilities as a member of CORE Response include coordination of outbreak response activities and providing subject-matter expertise for evaluation of data related to traceback investigations in support of FDA’s outbreak activities. Serving as one of CORE’s traceability subject-matter experts, she participates in interagency initiatives such as FDA’s “New Era of Smarter Food Safety,“ lending her expertise in developing and establishing traceback data metrics, revising field assignment templates, and serving as one of the leads developing the final rulemaking for the Food Traceability rule to implement FSMA Section 204(d)(1).
