PROCESS CONTROL
By Hal King, Ph.D., Managing Partner, Active Food Safety and James Marsden, Ph.D., Expert Advisor, Active Food Safety
New Uses for Existing Technologies to Reduce the Risk of Persistent Pathogens on Surfaces in Retail Foodservice and Sales Establishments
The need remains for a continuous means of sanitizing and disinfecting high-touch and other environmental surfaces where persistent microbial pathogens can be found

Image credit: fotogenicstudio/iStock / Getty Images Plus via Getty Images
SCROLL DOWN
Traditional environmental surface sanitation/disinfection in retail foodservice and sales establishments has been focused on food contact surfaces. However, persistent microbial pathogens like Listeria monocytogenes and Salmonella can survive on non-food contact environmental surfaces in retail foodservice facilities, leading to sporadic cases of foodborne illness through cross-contamination of foods. Likewise, viral pathogens like norovirus and Hepatitis A can survive and be transmitted from non-food contact environmental surfaces to foods, leading to large foodborne disease outbreaks. New uses for existing technologies are being deployed in retail foodservice and sales businesses to prevent these persistent pathogens where continuous sanitation/disinfection is needed.
Persistent Microbial Pathogens in Food Processing
A large number of microbial pathogens (including bacterial, viral, parasitic, and fungal) can persist in the environments found within a food processing facility. Some of these pathogens can not only survive harsh conditions in the environment (wide ranges of pH, temperature, and water activity) and resist chemical sanitation, but some (e.g., Listeria monocytogenes) can survive and grow under such conditions.1 This hardiness increases their probability of persisting in the environment of a manufacturing facility, therefore increasing the risk of causing foodborne illness to consumers if these pathogens then contaminate food products, especially ready-to-eat (RTE) foods during food processing.
One of the longest protracted foodborne illness outbreaks from a food manufacturing facility was caused by persistent Listeria monocytogenes that intermittently contaminated ice cream manufactured by Blue Bell Creameries company.2 The same strain of bacteria was found in the manufacturing facility that had been causing illness for over five years (Figure 1). The outbreak likely could have been prevented with the proper controls and monitoring to prevent persistent microbial pathogens in the manufacturing environment.
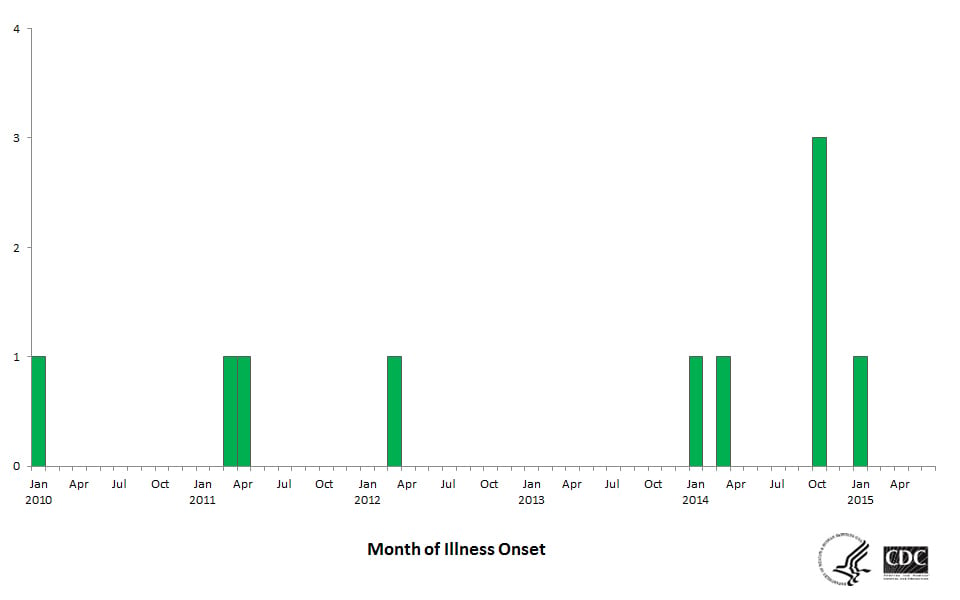
FIGURE 1. People Infected with the Outbreak Strains of Listeria monocytogenes, by Month of Illness Onset (n = 10 for Whom Information was Reported as of June 9, 2015) (Image Courtesy of CDC)
Due to the risk from persistent pathogens in RTE foods like the Blue Bell Creameries outbreak, the FDA rules prescribed by the Food Safety Modernization Act (FSMA) specifically require human food manufacturing businesses to monitor the control of environmentally persistent pathogens including Listeria monocytogenes during food processing. These rules require that a risk-based preventive control be established via a food safety plan for each food manufactured in a facility, and that microbial testing be performed via an environmental monitoring program (EMP).3 The recent investigation4 of foodborne illnesses and deaths linked to the bacterium Cronobacter sakazakii resulting from contaminated infant formula manufactured in a facility where this bacterium was also isolated shows that, although not as common as Listeria, other persistent microbial pathogens can be found in any food manufacturing facility.
Persistent Pathogens in Retail Foodservice and Sales Facilities
Persistent microbial pathogens can be found in retail foodservice and sales facilities, leading to foodborne illnesses and outbreaks after cross-contamination of foods. Listeria in retail deli environments has been associated with numerous foodborne illnesses and outbreaks.1 Sanitizer tolerance5 and biofilm formation are two contributing factors that lead to persistent Listeria in retail deli environments. Due to the prevalence of persistent Listeria in retail deli environments, USDA provides guidance6 to retail food businesses on how to control this persistent pathogen to prevent foodborne illnesses and outbreaks.
Other microbial pathogens can persist in retail foodservice and sales environments as well, leading to foodborne illnesses from continuous cross-contamination of foods over time. The CDC reported that a single restaurant7 was the source of a protracted, intermittent foodborne illness outbreak caused by the same strain of Salmonella enterica (Figure 2) for over ten years. During the time of each illness, these cases would have been considered sporadic cases.8 Interestingly, the same strain of persistent Salmonella enterica was found on several different environmental surfaces throughout the restaurant in two separate sampling/testing events, including cooking equipment in the kitchen area, preparation surfaces, dishwashing equipment, and storage areas. The pathogen was also isolated from employee restroom areas, where it was likely being shed by infected employees who touched these surfaces with their hands.
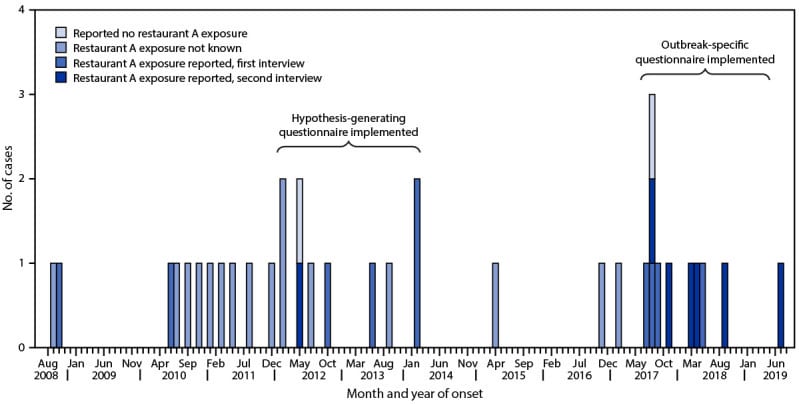
FIGURE 2. Cases of Salmonella Mbandaka Outbreak Subtype (n = 35), by Month and Year of Illness Onset and Restaurant A Exposure—Michigan, September 2008–July 2019 (Image Courtesy of CDC)
Notes: Onset date was missing for five patients; for these cases, the date of referral to the health department was used. Pulsed-field gel electrophoresis was performed only on the top 20 Salmonella serotypes submitted to the Michigan Department of Health and Human Services Bureau of Laboratories from approximately 2009 to early 2010; the Salmonella Mbandaka serotype was rare and not a top 20 serotype.
One of the most difficult challenges in the safe operation of a retail foodservice and sales business is preventing employees from working while sick. The greatest risk (from both the type of hazards and their high probability) is due to working sick employees with the infectious diseases Hepatitis A and norovirus.9 In fact, the most common cause of foodborne illnesses in foodservice establishments in the U.S. is due to norovirus.10 Norovirus and other foodborne viral pathogens could be considered persistent microbial pathogens because these viruses can continually contaminate and recontaminate surfaces via employees' hands (and gloved hands) and can survive for long periods of time on surfaces. Viruses like norovirus are often first transmitted in foodservice facilities via employees' hands to environmental surfaces like restroom door handles or handwash sink faucet handles.11 The viruses are unknowingly transmitted to other employees when they touch these common surfaces, which then leads to cross-contamination of foods, even when employees are wearing foodservice gloves.12
The foodservice business must include in its Food Safety Management System (FSMS) the Standard Operating Procedures (SOPs) to train and screen employees for illness in an attempt to prevent this first transmission event. However, because screening employees for illness is not 100 percent effective, the retail foodservice and sales business must also include the SOPs for environmental contamination controls similar to those used to prevent other persistent microbial pathogens.
How to Prevent Persistent Microbial Pathogens in Retail Foodservice and Sales Establishments
First, retail foodservice and sales facilities should be designed and constructed to ensure cleaning and sanitation efficacy and separation of raw and RTE food preparation and handling. The FDA Food Code and most state regulatory authorities include requirements for the cleanability of surfaces including floors (and grout between tiles), walls, ceilings, food preparation tables, food contact surfaces, sinks, etc. They also include restrictions on some surface types, such as floor carpeting. Equipment (ANSI-certified) placement in the design is also critical to ensure cleanability, especially where the surface meets the walls or other equipment/tables. Environmental surfaces in food preparation areas can harbor food debris, which can lead to the development of bacterial biofilms. All surfaces must be accessible and cleanable to remove all microbial pathogens, even if not a food contact surface. If these surfaces are neglected, they can lead to cross-contamination as the biofilms accumulate and are released on food contact surfaces or touched by employees, who can then transmit the biofilms to other food preparation surfaces or food.
Floors and equipment on floors are top areas in a restaurant for the persistence of pathogens, and improper use of reusable mops and mop water may contribute to this risk. Pathogens like Salmonella, Listeria, and norovirus can easily cross-contaminate other surfaces during body fluid cleanup procedures when they are aerosolized from employees walking on floors, the movement of wheels on carts, and during the mopping procedure. Thus, improper sanitation of floors and drains can contribute to the spread of pathogens that lead to foodborne illness outbreaks.
Second, each business should have a sanitation prerequisite program13 that is based on the type of hazards associated with the food processing procedures to prepare all menu items (e.g., marinating and preparing raw chicken or preparing raw ground beef). The most important elements of a sanitation prerequisite program are the sanitation procedures, the types of cleaning and sanitizing chemicals being used (e.g., do the sanitizing chemicals kill microbial pathogens like Listeria with the shortest amount of time), the training of employees and managers on proper procedures, and the evidence for proper execution by employees via monitoring using an FSMS. However, because not all cleaning and sanitization procedures are followed correctly or may not be executed as completely (e.g., on non-food contact surfaces where persistent microbial pathogens and norovirus can be found), there could be a need to enhance the sanitation prerequisite program with additional technology.
“As the pandemic wanes and businesses return to normal, there may be a perception that safety precautions are no longer needed to prevent COVID-19 where labor shortages and cost of labor are challenges; however, the risk of persistent microbial pathogens like norovirus are still prevalent.”


Elevating Sanitation Practices Using Technology
The cleaning tools, chemicals, and labor (training time included) needed to ensure that non-food contact surfaces are continuously cleaned and sanitized can be challenging. During the COVID-19 pandemic, it was critical to employee safety and the customer experience of safe14 within the retail foodservice and sales environments. However, as the pandemic wanes and businesses return to normal, there may be a perception that these precautions are no longer needed to prevent COVID-19 where labor shortages and cost of labor are challenges; however, the risk of persistent microbial pathogens like norovirus are still prevalent. The need remains for a continuous means of sanitizing and disinfecting high-touch surfaces and other environmental surfaces where persistent microbial pathogens can be found that could reduce chemical use and labor, as well as the associated risk of further transmission of these pathogens to the kitchen.
Several technologies based on germicidal ultraviolet (UV) light15 have been used in the past to disinfect air and surfaces in environments like hospitals and health care facilities, and during the pandemic these were used to effectively inactivate the SARS CoV-2 virus, the cause of COVID-19. These germicidal UV technologies can also improve surface sanitation and disinfection in food processing environments, including retail foodservice and sales facilities. One UV-based technology, photohydroionization (PHI), was widely used to help control SARS CoV-2 through a combination of the germicidal properties of the UV and the conversion of naturally occurring water vapor into airborne molecules of hydrogen peroxide.16
This conversion of water (H2O) into hydrogen peroxide (H2O2) occurs through a catalyzed oxidation reaction when UV energy reacts with water vapor in a specialized PHI device. H2O2 is an effective antimicrobial, and after it reacts and inactivates microorganisms in the air and on surfaces, it simply reverts to H2O. The levels of H2O2 produced by the PHI technology are very low and are similar to levels found in natural sunlight (0.02 ppm). These levels are well below government safety guidelines (1.0 ppm) and are safe in occupied spaces. Research has been conducted to validate the effectiveness of PHI technology on food and environmental surfaces. An evaluation of the effect of PHI technology to control Listeria on stainless steel surfaces, RTE cheese, and RTE turkey was reported previously. The results of the study17 indicated reductions (p < 0.05) in Listeria of 4.37 log colony forming units (CFU)/coupon on stainless steel after a 15-minute treatment and 1.39 log CFU/sample and 1.63 log CFU/sample after 120 seconds on RTE American cheese and RTE turkey, respectively. Germicidal UV sanitization/disinfection, using PHI technology, can be applied throughout retail foodservice and sales environments to provide continuous reduction of microbial pathogens like norovirus on high-touch surfaces in public restrooms. It has been widely used in some foodservice businesses18 as an additional control of microbial pathogens.
Direct germicidal UV-C light (where the UV source is focused on a surface) is one of the most effective ways to kill surface and airborne pathogens, and this technology has been used in hospitals for many years. However, germicidal UV-C light is not safe to use around people due to the potential harm to eyes and skin, which means it can be used only on environmental surfaces when people are not present. Far UV-C, on the other hand, is an emerging safe UV technology19 (200–230 nm) that does not harm the skin or eyes, unlike traditional germicidal UV-C, providing a safe way to reduce pathogens in the areas where people work and eat. Far UV-C has been shown to inactivate and kill several pathogenic bacteria and viruses[achieving more than 4-log (99.99%) reduction for most of the pathogens], including waterborne, foodborne, and respiratory pathogens in a thin-film aqueous solution.20
This technology has also been tested for the reduction of microbial pathogens in air using surrogate bacteria suspended in air21 and the SARS CoV-2 virus on surfaces.22 The use of Far UV-C to inactivate microbial pathogens on RTE foods is the topic of a current USDA-funded research project.23 In a controlled field test of Far UV-C24 for the disinfection of viruses in an occupied office setting, complete inactivation of 6 log (99.9999%) of the Phi6 bacteriophage virus was achieved in approximately 20 minutes of exposure time. To achieve this efficacy, the researchers modeled the combined precise irradiance distribution and coverage to generate the correct dosage on the surface.
Modeling for efficacy against persistent microbial pathogens like Listeria or human norovirus on retail foodservice and sales surfaces would be needed to validate the technology, but the use case and value in reducing these risks could be significant. For example, a Far UV-C device could be used to reduce persistent microbial pathogens like norovirus on high-touch surfaces to augment a sanitation prerequisite program by placing the devices in restrooms to "cover" high-touch surfaces, especially those identified by FDA as key touchpoints for transmission of norovirus: restroom door handles (inside), restroom faucet handles (each sink), and restroom toilet stall door latches. Far UV-C devices, modeled to focus on surfaces with a distance and efficacy for eliminating norovirus on high-touch surfaces, could reduce the cleaning tools, chemicals, and labor needed to ensure that these environments are continuously sanitized, especially as they are continuously used by both employees and customers.
Takeaway
Environmental surfaces found in retail foodservice and sales establishments, including food contact surfaces, will always need to be cleaned first and then sanitized afterward to maximize the efficacy of removing persistent microbial pathogens. In fact, it is a requirement in the FDA Food Code to first clean and then sanitize a food contact surface to ensure sanitation and prevent cross-contamination of microbial pathogens from food contact surfaces to food. However, non-food contact surfaces that have been cleaned may not have the same degree of soil (i.e., they look clean), even as the surfaces are continuously touched by hands and possibly recontaminated with microbial pathogens multiple times during businesses operating hours. Therefore, the potential for using technology to augment retail foodservice and sales establishments' ability to reduce the risk of persistent microbial pathogens may be important.
Acknowledgement
The authors thank Megan Groves of Population Tech and Dr. Jasdeep Saini of WTI Inc. for their technical input.
References
- Forauer, E., S. T. Wu, and A. J. Etter. "Listeria monocytogenes in the retail deli environment: A review." Food Control 119 (2021): 107443. https://www.sciencedirect.com/science/article/abs/pii/S0956713520303595.
- Centers for Disease Control and Prevention (CDC). "Multistate Outbreak of Listeriosis Linked to Blue Bell Creameries Products (Final Update)." June 10, 2015. https://www.cdc.gov/listeria/outbreaks/ice-cream-03-15/index.html.
- King, H. and W. Bedale. Hazard Analysis and Risk-Based Preventive Controls. Cambridge: Academic Press, October 2, 2017. https://www.elsevier.com/books/hazard-analysis-and-risk-based-preventive-controls/king/978-0-12-809475-4.
- CDC. "Cronobacter and Powdered Infant Formula Investigation." May 24, 2022. https://www.cdc.gov/cronobacter/outbreaks/infant-formula.html.
- Møretrø, T., B. C. T. Schirmer, E. Heir, et al. "Tolerance to quaternary ammonium compound disinfectants may enhance growth of Listeria monocytogenes in the food industry." International Journal of Food Microbiology 241 (January 2017): 215–224. https://www.sciencedirect.com/science/article/pii/S0168160516305621.
- U.S. Department of Agriculture Food Safety and Inspection Service (USDA FSIS). "Guidance for Controlling Listeria monocytogenes (Lm) in Retail Delicatessens—Best Practice Tips for Deli Operators." May 25, 2016. https://www.fsis.usda.gov/inspection/compliance-guidance/microbial-risk/guidance-controlling-listeria-monocytogenes-lm-retail.
- Nettleton, W. D., B. Reimink, K. D. Arends, et al. "Protracted, Intermittent Outbreak of Salmonella Mbandaka Linked to a Restaurant—Michigan, 2008–2019." Morbidity and Mortality Weekly Report 70, no. 33 (August 20, 2021): 1109–1113. https://pubmed.ncbi.nlm.nih.gov/34411074/.
- CDC. "Attribution of Foodborne Illness: Methods and Data Sources." November 5, 2018. https://www.cdc.gov/foodborneburden/attribution/data-sources.html.
- King, H. "Breaking the Chain of Infectious Disease Transmission in a Retail Foodservice Business." Food Safety Magazine. August 17, 2020. https://www.food-safety.com/articles/6750-breaking-the-chain-of-infectious-disease-transmission-in-a-retail-foodservice-business.
- CDC. "Annual Summaries of Foodborne Outbreaks." September 12, 2019. https://www.cdc.gov/fdoss/annual-reports/index.html.
- U.S. Food and Drug Administration (FDA). "Risk Assessment of Norovirus Transmission in Food Establishments." March 6, 2023. https://www.fda.gov/food/cfsan-risk-safety-assessments/risk-assessment-norovirus-transmission-food-establishments.
- King, H. and B. Michaels. "The Need for a Glove-Use Management System in Retail Foodservice." Food Safety Magazine. June 18, 2019. https://www.food-safety.com/articles/6258-the-need-for-a-glove-use-management-system-in-retail-foodservice.
- King, H. "Sanitation Prerequisite Programs as a Necessary Component of FSMS for Foodservice Establishments." Food Safety Magazine. October 11, 2022. https://www.food-safety.com/articles/8055-sanitation-prerequisite-programs-as-a-necessary-component-of-fsms-for-foodservice-establishments.
- Deloitte. "Deloitte: The Restaurant of the Future Should Evolve to Serve Consumers in New Ways." December 8, 2021. https://www2.deloitte.com/us/en/pages/about-deloitte/articles/press-releases/deloitte-the-restaurant-of-the-future-should-evolve-to-serve-consumers-in-new-ways.html.
- Illuminating Engineering Society. "IES Committee Report: Germicidal Ultraviolet (GUV)—Frequently Asked Questions." April 15, 2020. https://media.ies.org/docs/standards/IES-CR-2-20-V1-6d.pdf.
- Fink, R. G. and W. B. Ellis. "Device, system and method for an advanced oxidation process using photohydroionization." Google Patents. Patent No. US7988923B2. https://patents.google.com/patent/US7988923B2/en.
- Saini, J. K., J. L. Marsden, K. J. K. Getty, and D. Y. C. Fung. "Advanced oxidation technology with photohydroionization as a surface treatment for controlling Listeria monocytogenes on stainless steel surfaces and ready-to-eat cheese and turkey." Foodborne Pathogens and Disease 11, no. 4 (April 2014): 295–300. https://pubmed.ncbi.nlm.nih.gov/24444302/.
- "Chipotle Takes Another Step to Improve Food Safety." QSR Magazine. January 10, 2017. https://www.qsrmagazine.com/news/chipotle-takes-another-step-improve-food-safety.
- International Ultraviolet Association (IUVA). "Far UV-C Radiation: Current State-of Knowledge." https://iuva.org/resources/covid-19/Far%20UV-C%20Radiation-%20Current%20State-of%20Knowledge.pdf.
- Ma, B., K. Bright, L. Ikner, et al. "UV Inactivation of Common Pathogens and Surrogates Under 222 nm Irradiation from KrCl* Excimer Lamps." Photochemistry and Photobiology (September 21, 2022). https://pubmed.ncbi.nlm.nih.gov/36129750/.
- Eadie, E., W. Hiwar, L. Fletcher, et al. "Far-UVC (222 nm) efficiently inactivates an airborne pathogen in a room-sized chamber." Nature: Scientific Reports 12, no. 4373 (March 23, 2022). https://www.nature.com/articles/s41598-022-08462-z.
- Kitagawa, H., T. Nomura, T. Nazmul, et al. "Effectiveness of 222-nm ultraviolet light on disinfecting SARS-CoV-2 surface contamination." American Journal of Infection Control 49, no. 3 (March 2021): 299–301. https://www.sciencedirect.com/science/article/pii/S0196655320308099.
- USDA Research, Education, and Economics Information System (REEIS). "Microplasma-Based Far-UVC Light Decontamination Approaches for Improving the Safety of Ready-to-Eat Meat Products." Wang, Y. 2022. https://portal.nifa.usda.gov/web/crisprojectpages/1027776-microplasma-based-far-uvc-light-decontamination-approaches-for-improving-the-safety-of-ready-to-eat-meat-products.html.
- Seyedi, S., B. Ma, M. Groves, H. King, and K. G. Linden. "Field Study and Evaluation of KrCl* Far UV-C Device Capability for Inactivation of Phi6 Bacteriophage." Photochemistry and Photobiology (December 19, 2022). https://pubmed.ncbi.nlm.nih.gov/36533876/.
Hal King, Ph.D. is Managing Partner of Active Food Safety, www.activefoodsafety.com, and a member of the Editorial Advisory Board of Food Safety Magazine. He can be reached at halking@activefoodsafety.com.
James Marsden, Ph.D. is an Expert Advisor at Active Food Safety.

