COVER STORY

Tackling the Challenges of Microbial Challenge Studies
Defining the objective of a challenge study may be less straightforward than it may appear initially
SCROLL DOWN
Video credit: tawattiw/Creatas Video via Getty Images
By Kathy Glass, Ph.D., Associate Director, Food Research Institute (FRI), University of Wisconsin–Madison; Kristin Schill, Ph.D., Assistant Research Professor, FRI, University of Wisconsin–Madison; and Cindy Austin, Ph.D., BSL-2 Lab Manager, Meat Science and Animal Biologics Discovery, FRI, University of Wisconsin–Madison
Imagine the following scenario: your product developers plan to launch a new refrigerated meal in four months, and they need a validated food safety plan. There has been a recent outbreak in a product that is similar to yours, suggesting that your product may be at risk for a pathogen that was not previously considered "reasonably likely to occur." Alternatively, you discover that processing for a product has changed considerably since a validation study was conducted 20 years ago. Yikes! Where should you start? Who do you call for help?
While this brief article is not intended to be a substitute for working with a food microbiologist with expertise in microbial challenge studies, it offers an outline of considerations and resources to consult.
Types of Challenge Studies
Defining the objective of the study may be less straightforward than it may appear initially. The two types of challenge studies are pathogen growth inhibition and pathogen inactivation. It is possible that your product may need both studies conducted. The choice of target pathogen(s), inoculation method, inoculation level, temperature(s) tested, and controls will vary depending on the study objective(s). Can you find "safe harbors" in regulations, validated predictive models, or published literature that sufficiently match your product to substitute for a challenge study, or will you be able to reduce the number of experiments needed for validation?
Information Needed Before Designing a Study
It is important to understand the product formulation, processing, any relevant regulatory requirements, and intended and unintended storage and handling at retail and by the consumer. Gather as much detail as possible. Certain information may already be known from production records of existing or similar product, whereas other types of information may require testing of prototype product.
Marketing may have a target shelf life in mind, but product developers may not have the data to support a product formulation that will last the duration of the desired shelf life. The type of spoilage and time to unacceptable quality under ideal storage conditions, as well as the consequences of product temperature abuse, are essential parameters in determining the length of the study and what controls are required.
Know Your Production Ranges for pH and Water Activity
Normal production of a given food product will have a target and acceptable ranges for intrinsic control parameters, such as pH, moisture, and salt. Since pH and water activity are two driving factors in determining microbial growth, collecting data on these factors over time (and even within lots produced) will determine the appropriate upper and lower limits used in product testing (i.e., "worst-case scenario"). The effect of these parameters can affect the type and design of the study, as well as the expected outcome.
Lower water activity results in reduced microbial growth rate. Conversely, reduced water activity or higher fat is associated with slower inactivation rates during thermal or high-pressure processing (HPP). Therefore, testing at the uppermost moisture and/or water activity is recommended for a growth inhibition study, whereas product samples with lower moisture or water activity are more appropriate for an inactivation study.
Optimal growth for most microbes is pH 5.0–7.5. As the pH diverges to the acidic or alkaline ranges, growth is delayed and inactivation is accelerated, particularly at higher storage or cooking temperatures. Foods where pH is intended to be the primary safety driver should be tested with the lowest concentration of acid and, therefore, a higher pH. For example, if the target pH of cream cheese is 4.9, but the maximum encountered is pH 5.1, then a growth inhibition study should be conducted on the product with a pH of slightly greater than the upper limit (e.g., testing at pH 5.2). Thermal inactivation rates of Salmonella in egg white with pH 9.1 may be quicker than at pH 8.2.1 Studies may be needed on multiple product types to account for the effect of pH.
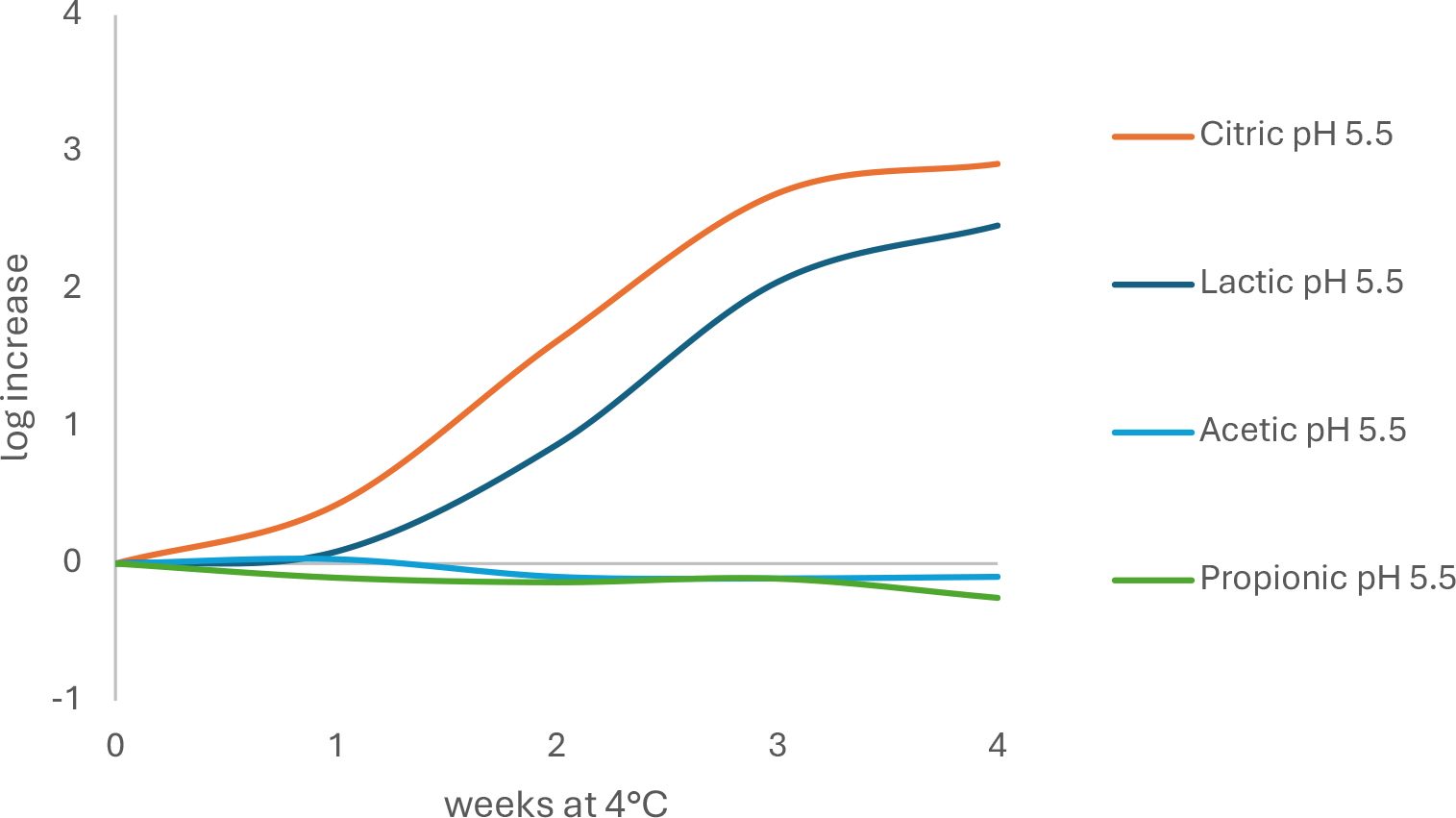
Not only is pH important, but the type of acidulant that is used can exert effects beyond that of reducing the pH. The minimum pH value for growth of a particular pathogen that is frequently reported in the literature is based on hydrochloric or citric acid. If challenge testing is done on a product formulated with lactic acid or vinegar (acetic acid), then the minimum pH for growth may be higher than if lemon juice (citric acid) is used to adjust pH. Therefore, changes in acidulant may require additional challenge tests (Figure 1).
FIGURE 1. Differences in growth rate of L. monocytogenes in model soft cheese (56 percent moisture, 1.25 percent salt) made with different acids and stored at 4 °C for 4 weeks (adapted from Engstrom et al.2)
Other details needed to design the challenge study include:
- Product description, including ingredients and normal microbial load and species that can be found in the raw ingredients and finished product. How consistent are the ingredients from various sources and from lot to lot? Ingredients that previously have had a microbial reduction step, such as pasteurization, validated carcass washes, or refined ingredients, have different species and levels of indigenous microorganisms than those that inhabit raw peeled root vegetables where spore-forming bacteria may predominate. Growth of background microbiota can result in acid production and compete with pathogens. Failure to consider the variation in spoilage characteristics can lead to falsely concluding that a product is safe.
- Physiochemical characteristics: What are the moisture, salt, water activity, pH, acid type, and fat levels of the composite sample and of each individual component, if applicable? Are these values measured or calculated? Do any of the values for physiochemical properties change from preparation to consumption? This is particularly true of pH changes due to the growth of spoilage microbes, or changes in available moisture or pH at the interface of multiple components due to equilibration. Shifts can be influenced by storage temperature and packaging permeability.
- What are the preparation steps, lethality steps, cooling, and potential for re-contamination?
- How long does it take from the start of production to packaging? Do extended runs need to be validated to ensure no production of heat-stable enterotoxin by Staphylococcus aureus or Bacillus cereus?
- Is there a heating step or cooling step that needs validation? Can regulatory requirements be used as safe harbors? Do your product and process match those covered by the regulatory guidelines?
- Is the product hot-filled or cooked-in-bag so that vegetative pathogens are eliminated? Can the food tolerate a "nonprot Bot cook" (equivalent to 90 °C for 10 minutes to reduced populations of Group II psychrotrophic Clostridium botulinum spores by 6 logs)? This will eliminate the need to test a group of C. botulinum, but still require tests with B. cereus or Group I (mesophilic) C. botulinum. Is the product exposed to the environment after the lethality step? Is L. monocytogenes testing needed?
- Does the formulation include food ingredients that have growth inhibitory properties, either synthetic or clean-label antimicrobials? Why was the preservative chosen? Nitrite is a potent inhibitor to anaerobic Clostridia, B. cereus is particularly sensitive to nisin, and propionate delays growth of L. monocytogenes more consistently than it affects Clostridia. Other organic acid salts (lactate, diacetate, sorbate) typically inhibit most Gram-positive pathogens, but to varying degrees. Synthetic antimicrobials have defined and consistent activity, whereas cultured sugar-vinegar products are frequently proprietary blends of organic acids and potentially antimicrobial peptides. If ingredients are changed due to cost or their effect on sensory properties, then challenge studies may need to be repeated.
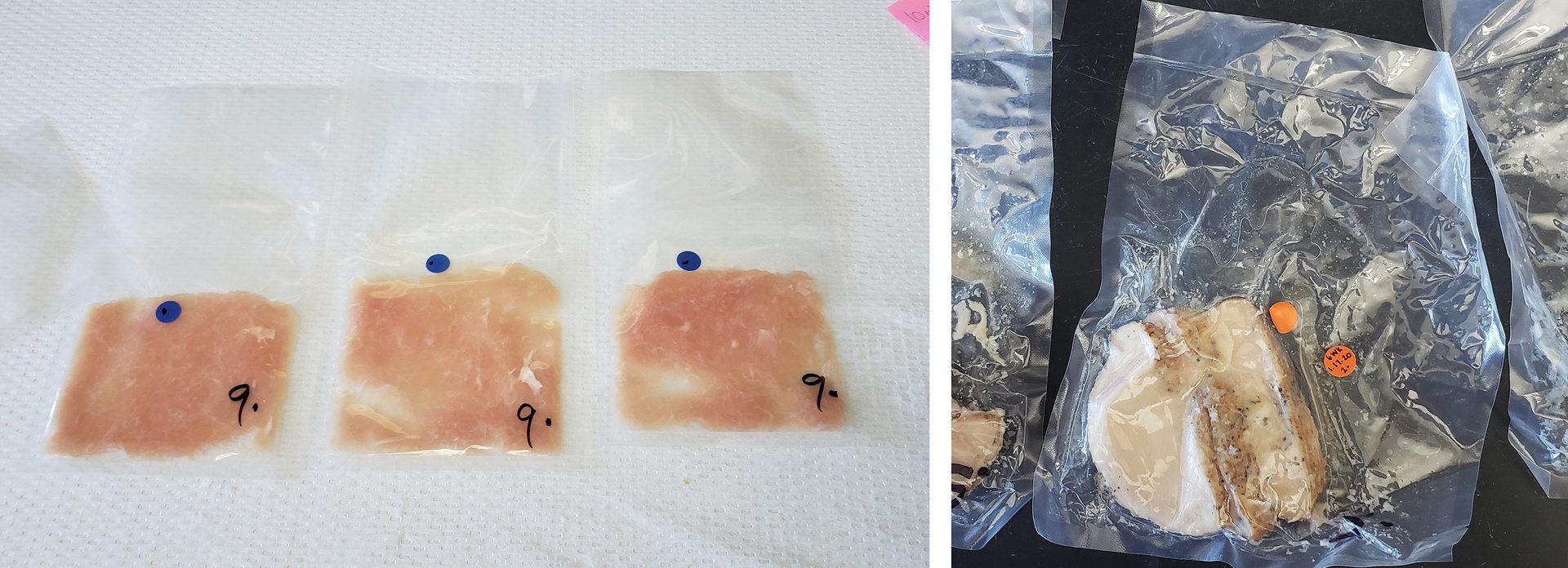
- How are the antimicrobials applied? Ground or homogenous matrices ensure rapid equilibration and consistent delivery of antimicrobials throughout the product matrix. However, product such as tumble-marinated meat may not have the same level of salt or other antimicrobial in the center of the meat compared to the outside edge. This can result in inconsistent growth inhibition and potential false results depending on whether the microbe is in contact with the antimicrobial (Figure 2).
FIGURE 2. Product form can result in differences in antimicrobial distribution
- Is the food matrix homogenous, or is it a multi-component food? A homogeneous matrix will deliver more consistent microbial growth or inactivation rates between replicate samples or trials. Slow equilibration in water activity and pH among distinct layers may result in microenvironments in which microbes can unexpectedly grow. Multi-component foods may require additional replication to account for variability (Figure 3). Inoculation should be at interfaces where equilibration is a factor or where the pathogen is most resistant to inactivation (such as if seeds are exposed to surface drying during baking).
FIGURE 3. Multicomponent foods may require multiple inoculation sites and testing

- Packaging: Although Clostridium perfringens and C. botulinum are classified as anaerobes and are preferentially selected for by vacuum or reduced-oxygen packaging (ROP), the presence of aerobic conditions, such as oxygen on the product surface, is not a guarantee that these spore formers will not grow in the interior of the package. For seafood, packaging must have sufficient oxygen permeability to allow naturally occurring spoilage microorganisms to grow and spoil product before C. botulinum can grow and produce toxin. Spoilage for other food commodities will be different. Furthermore, most foodborne pathogens, such as L. monocytogenes or Bacillus cereus, are facultative anaerobes. While their growth rate might be slightly faster under aerobic conditions, they can grow almost as well in ROP. Some foods may require more than one psychrotrophic pathogen to be tested because a given strategy that controls one pathogen may not be adequate to control another.
- What is the potential temperature during distribution, display, storage at retail and consumer levels, anticipated use, and potential abuse? A 2007 survey showed that 5 percent of food in consumer refrigerators are stored at temperatures higher than 7 °C, with 0.5 percent of food higher than 10 °C; 35 percent of deli counter meats were stored at temperatures higher than 7 °C.3 At this temperature, Listeria populations can increase by greater than 2 logs within 1 week. Foods that are intended for children may be held for hours or days at ambient temperature if they are in lunch packs or used for "on-the-go" snacks. Epidemiology shows that most outbreaks of botulism are associated with foods stored at nonrefrigerated conditions, even if they are labeled as "keep refrigerated." Therefore, "disaster testing," such as incubation at room temperature for several days or until gross spoilage, may be appropriate for certain foods.
- Are there multiple similar formulations in the portfolio? What are the key parameters of the formulation related to safety? Can a select number of formulations be used to represent the entire portfolio? Challenge studies are time- and resource-intensive, and replication is needed to ensure that data will represent variation in production, as well as microbial populations that may contaminate the food. In some cases, product formulations are similar enough that representative SKUs can be used as replicates. However, it is important to scrutinize the ingredients and controls to avoid situations like those described below for the two soups (Table 1).
Controlling Product Spoilage
One frequently overlooked factor is the type and time to microbial spoilage. Color fading or oxidative rancidity may affect the "best by" date, but has no bearing on microbial quality and safety ("use by" date). In general, control of microbial spoilage is more difficult than inhibition of pathogenic microorganisms and depends on the use of good-quality ingredients and sanitation, and less on antimicrobial ingredients.
FIGURE 4. Comparison of the growth of a spoilage microbe, Leuconsotoc mesenteroides, vs. L. monocytogenes at 4 °C in a model uncured deli-style turkey, 70 percent moisture, 3.75 percent cultured sugar (adapted from Weyker et al.4)
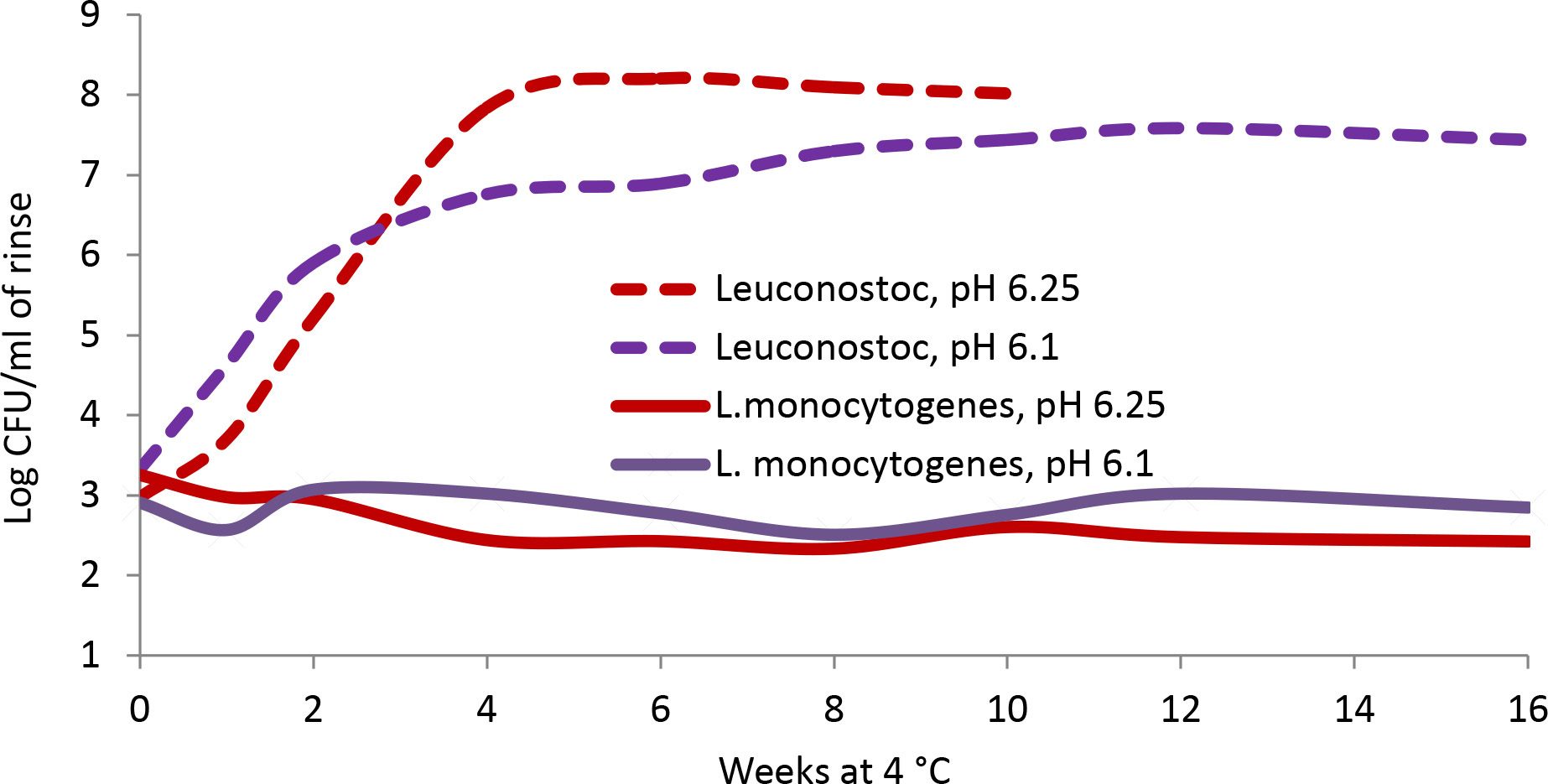
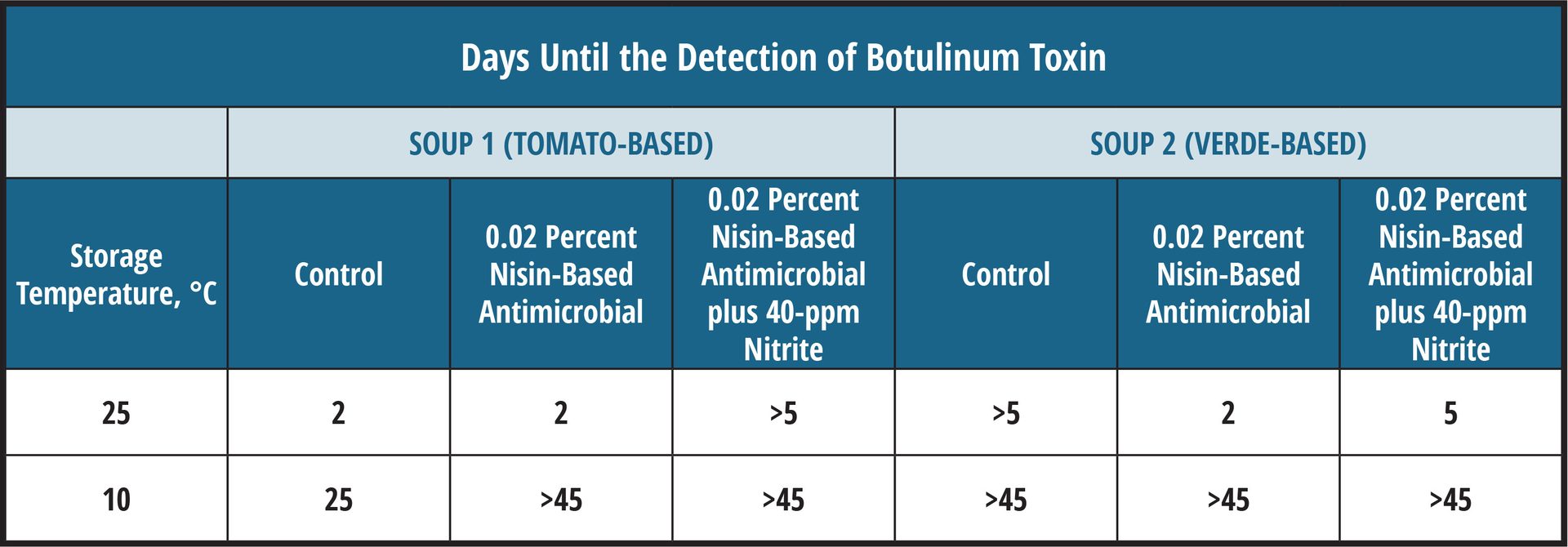
In contrast, other spoilage microbes may be more sensitive than pathogens to certain antimicrobials. Table 1 shows the time to toxicity for two soup varieties with similar pH and antimicrobial addition that had been inoculated with C. botulinum. Differences in the soups were the choice of vegetables and the broth base.
In the case of the tomato-based soup, the addition of nisin alone inhibited spoilage (no change in pH during test period) but was not more effective than the no-antimicrobial control for inhibiting C. botulinum when the product was stored at room temperature. When nisin was used in combination with nitrite, botulinum toxin production was inhibited until spoilage, suggesting the combination formulation would provide the best protection.
In contrast, spoilage microbes grew rapidly in the control verde-based soup, decreasing the pH to less than 4.8 within 3 days. The acid production in the control resulted in greater inhibition of C. botulinum than in soups with nisin where spoilage was inhibited. When stored at moderate abuse temperature (10 °C), antimicrobials were effective against both the target pathogen and spoilage microbes.
This study emphasized the need for testing controls and various temperatures, and monitoring spoilage to determine the best management system.
TABLE 1. Effect of antimicrobials (nisin, nitrite derived from cultured celery powder) on spoilage and on C. botulinum in soups (high-pressure pasteurized to kill vegetative spoilage microbes; initial pH 5.2–5.3)
Safe Harbors
Before deciding to invest in a challenge study, check whether your product may be covered by existing data. Acidified foods with an equilibrated pH of less than 4.6 throughout the matrix are known to inhibit growth of C. botulinum, as well as many other foodborne pathogens, and are the basis of low-acid canned food regulations.5 These products will still need a lethality step (such as a hot-fill or acid-hold) to ensure elimination of low infectious dose, acid-tolerant pathogens such as Escherichia coli O157:H7.
A review of data collected for numerous challenge studies suggest that combinations of pH less than 5.6 and water activity less than 0.95 are sufficient to inhibit the growth of pathogenic, spore-forming bacteria.6,7 While this is a good guideline to evaluate growth, it may not be sufficient by itself for compliance with U.S. low-acid canned food regulations.
Using Predictive Models
Predictive growth and inactivation models can be useful tools to discern the appropriate target pathogen(s) for a given study based on the intrinsic properties of the food (namely pH, water activity, and salt content) and temperature at which the food is intended to be stored. Additionally, these mathematical models can shed light on how well the target pathogen(s) are predicted to grow within the food of interest and can be helpful when determining the sampling intervals. These models are often based on data collected in a microbiological growth medium, but some models are based on data collected in food matrices.
It is important to understand the limitations when using any given model. Although models can be used to justify the decision not to conduct a challenge study, careful consideration of how well the model fits the food product and growth parameters for the target pathogen is warranted. Several online, user-friendly models include ComBase,8 the Agricultural Research Service Pathogen Modeling Program (PMP),9 Food Spoilage and Safety Predictor,10 and the Danish Meat Research Institute (DMRI) predictive models.11 Ingredient suppliers may also offer portfolio-specific models that require registration for access.
Temperature Choices
Storage temperature for a growth challenge study should mimic the expected temperatures during a product's distribution. The National Advisory Committee on Microbiological Criteria for Foods (NACMCF) recommends 7 °C for refrigerated foods to account for how consumers store products in the U.S.3,6 Refrigerated studies may include other temperatures, like 4 °C, which is the ideal temperature for food safety, or 10–12 °C if product is being sold in countries where refrigeration practices are higher. Depending on the expected ambient temperatures and effect on deterioration of the product, shelf-stable products should be stored in the range of 24–35 °C for challenge studies.
For validation of inactivation of thermal or high-pressure treatments, studies should include temperatures or pressures and times that could represent process deviations, to demonstrate that critical limits have a margin of safety. Although time- and resource-intensive, studies that create D- and z-values for a given food matrix offer the greatest flexibility in designing a process. They also provide documentation for evaluating process deviations, including cooking and hot-fill deviations. This may include testing a minimum of four temperatures and 5–7 pull times.12,13 Calculated values can then be used in a spreadsheet to determine log kill under various time–temperature conditions.14
Choosing the Right Microbe(s)
The appropriate microorganism(s) for challenge studies should be determined by an expert microbiologist and based on several factors. This determination includes the type of product and its intrinsic characteristics (e.g., pH and aw), whether the product has a lethal processing step and what type, and which pathogens are associated with that product type. Resources to consult include the NACMCF document,6 the Food and Drug Administration (FDA)15 or U.S. Department of Agriculture (USDA) Hazard Guides, the International Commission on Microbiological Specifications for Foods' (ICMSF's) "Microorganisms in Foods 5: Characteristics of Microbial Pathogens,"16 the Institute of Food Technologists' (IFT's) "Definition and Evaluation of Potentially hazardous Foods,"7 the FDA Food Code,17 predictive models, and published literature.
Testing of all potential pathogens is unnecessary, but more than one pathogen may be relevant. For growth studies, determine which microorganism(s) can grow the fastest under the specified storage conditions and is the most likely to be present. For inactivation studies, choose the microorganism that is the most resistant to the lethal step (e.g., heat, HPP, antimicrobials, acidify and hold, acid/drying combined) and is the most likely to be present.
The choice of strains used in the inoculated pack study are often as important at the genus and species themselves. Ideally, the strains chosen should be food, clinical, and/or environmental isolates relevant to the food to be challenged, with known growth characteristics in terms of minimum growth temperature and pH, as well as tolerance to other food or processing conditions. For example, while some strains of B. cereus are psychrotrophic, growth might occur only at temperatures greater than 8 °C; therefore, incubation at 4 °C may not be relevant to test the robustness of the formulation's inhibitory properties.
The American Type Culture Collection (ATCC), the UK National Collection of Type Cultures (NCTC), and the New Zealand Reference Culture Collection are examples of collections that have isolates. Extensive study of the collections may be required to find the primary source of the individual strains and determine if their growth characteristics match the targets for the study. Some laboratories have collected appropriate isolates from various sources over the years, but they may not be able to share with other laboratories due to shipping restrictions for certain pathogens. Alternatively, Cornell University has been chosen by the Food Microbiology Committee of the Institute for the Advancement of Food and Nutrition Sciences (IAFNS) to stock appropriate strains of L. monocytogenes and Salmonella for research, including for challenge studies.
"All studies should include controls. Uninoculated controls are important in understanding if products will spoil, regardless of whether or not pathogens are present."


Cultures used in challenge studies are typically grown to stationary phase in nonselective media and should be prepared to develop the greatest resistance to the conditions to be tested. For example, E. coli O157:H7 should be acid-adapted prior to inoculation in low-pH foods.18 Culturing pathogens on solid agar has been shown to enhance the resistance of Salmonella for studies on thermal inactivation in low-water-activity foods.19,20 To reduce the effect of moisture added with cultures to low-moisture foods, the use of talc as a carrier for Salmonella (or its surrogates) has been described.21
Pathogenic bacteria are typically found at levels of 2 log CFU/g or lower, and frequently at lower than detectable levels if using a 25-g sample. To obtain reliable counts for enumeration, it is recommended that foods used for growth inhibition studies be inoculated with 2–3 log CFU per g or per ml. This allows more accurate enumeration for zero-time inoculated samples and consistent distribution in the food matrix, but still allows a 4–5-log increase before the cells reach stationary phase. For inactivation studies targeting a 5-log reduction or greater, inoculation with 7 or 8 log CFU/gram is appropriate.
In some validation processes, the use of equipment such as specialized ovens, smokehouses, or roasters cannot be replicated in a Biosafety Level 2 (BSL-2) laboratory. Surrogates have been successfully used in a variety of applications,22 but it is important that the surrogate be validated for that particular circumstance (e.g., humidity, type of heating, and food matrix).
Controls and Replication
All studies should include controls. Uninoculated controls are important in understanding if products will spoil, regardless of whether or not pathogens are present. Also, uninoculated controls can indicate how other competitive organisms will grow in the presence of pathogens, or how they are affected by formulation. A negative growth control is useful to set a no-growth boundary, and a positive control indicates that the pathogen would grow under permissive conditions. Either or both may be necessary to establish critical production limits.
Is a Lab-Tested Product Representative of Production?
Commercial and academic labs may have the capabilities to work with the target pathogenic bacteria, but may not have food production equipment to mimic product formulation and processing. The producer may need to use pilot production facilities to provide finished product that represent "in spec" and "out of spec" for inoculation and testing.
As suggested earlier, the formulation for growth inhibition studies or conditions tested for lethality will become the critical control limits for production. Values such as moisture, pH, and salt may be relatively straightforward to control in some products, but more challenging in others due to variation in incoming ingredients. For example, a product may be safe at the production target of 70 percent moisture and pH 6.2, but a higher moisture and pH that represent a range of production may not have the same inhibitory properties. Therefore, the CCPs will either need to comply with the limits tested in the laboratory, or additional lots must be tested to ensure that production ranges are covered. The location of inoculation should mimic where the microbe is most likely to occur. Post-process contamination by L. monocytogenes can be delivered as a surface inoculum, whereas spores can be incorporated into the matrix to represent the internalization that occurs during mixing, injection, or another process.
Defining Growth Inhibition and Log Reduction Needed
Manufacturers should consult regulatory guidance for appropriate microbial target and log increase or reduction for their product. The NACMCF identified a 1-log increase as a typical pass/fail benchmark for growth and recommends testing for 1.25 times shelf life.6 Codex provides a definition for a food supporting the growth of L. monocytogenes, in which there is greater than an average of 0.5 log CFU/g increase in L. monocytogenes populations for the expected shelf life of the food, under reasonably foreseeable conditions of distribution, storage, and use (anticipated storage temperatures of 6 °C).23
Canada limits growth of L. monocytogenes to no more than a 1-log increase when stored at 7 °C,24 while New Zealand recognizes growth as no greater than a 0.5 log increase.25 USDA's Food Safety and Inspection Service (FSIS) defines specific requirements for pathogen log reduction26,27,28 or growth limits29, 30 in meats and eggs.31 FDA is less prescriptive for many food categories, with the exception of juices32 and almonds,33 although a 5-log reduction is a commonly accepted target for many foods.
Interpreting Results
Expert microbiologists should interpret the results of a microbiological growth or inactivation study, as this process is rarely as simple as comparing initial and final counts. Numbers from different sampling points may vary due to inherent errors in sampling and enumeration methods, so it might be difficult to determine if changes in numbers are real or due to analytical variability.
There are several considerations to watch out for when interpreting results for growth studies. Initial die-off after inoculation, followed by growth that does not exceed the target inoculation, may not be recognized. This may be addressed by allowing an equilibration time before enumerating initial levels. Normal sample variation may cause a spike at a sampling interval that may not be significant, particularly in multicomponent foods. Testing representative samples of multiple units will address this concern. Graphical representation of the data can be useful in assessing whether actual growth occurred. Statistically significant differences between samples at one sampling point or between replicate experiments can be an indication that either the inoculation method was inconsistent, or the food environment is at the boundary of growth/no growth.
An increase in one log cycle over two or more time intervals is generally considered significant by expert microbiologists. Smaller increases may be significant depending on the enumeration methods, number of samples, and replicates used.
Pass-fail criteria for toxin formers are often based on the detection of toxins. No toxin detection in growth studies conducted with C. botulinum should be the criteria, as toxin production can occur without an observable increase in populations due to difficulties in enumeration, particularly for Group II C. botulinum. However, limiting growth of S. aureus to less than 3 log CFU/g may be used as the failure criteria as an alternative to enterotoxin testing.
"Time constraints, budget, availability of qualified labs, and pressure from marketing, product developers, or customers to deliver the 'right answers' can interfere with designing and conducting a study that delivers accurate and reliable results."


Lethality experiments should meet any existing regulatory requirements and should be determined through replicate trials. Where no performance standard exists, the lowest log reduction achieved should exceed the expected contamination level (typically a maximum of 2–3 log CFU/g or per ml for raw product) by an amount that incorporates a margin of safety (typically a 2-log margin) consistent with the variability expected in the product and the process.
Choosing a Laboratory to Run a Study and Qualifications Needed
The ideal laboratory (commercial or academic) will be authorized to work with a variety of BSL-2 foodborne pathogens and have the following:
- An extensive stock culture collection with relevant stains with robust growth characteristics
- Dedicated pilot-scale food processing and packaging equipment
- A variety of reliable incubators
- Equipment for proximate analysis (e.g., moisture, pH, salt, water activity, etc.)
- Expertise in designing, conducting, and interpreting food challenge studies.
The laboratory may not be the same one that you would choose for routine microbial testing, but it should use accepted methods, such as cultural methods found in FDA's Bacteriological Analytical Manual,34 FSIS,35 or peer-reviewed publications. Few laboratories in North America are authorized to work with C. botulinum in foods, so choices are limited. Check publications on the food–pathogen pair of interest, and ask your network of professional contacts or inspector for recommendations.
Cautions
Time constraints, budget, availability of qualified labs, and pressure from marketing, product developers, or customers to deliver the "right answers" can interfere with designing and conducting a study that delivers accurate and reliable results. While an expert microbiologist will work with you to make the study efficient, cost-effective, and to cover the greatest number of contingencies, foods are complex, and results may not always support the predictions. Even the most experienced lab may not be able to predict sporadic spoilage that may invalidate the study or how microenvironments will affect growth or inactivation.
Tested parameters will define CCP limits. Include tests that represent slightly "out-of-spec" conditions in the event that production limits are not met. This may make the difference between releasing the product after a thorough review by your process authority vs. landfilling a day's worth of production. Keep in mind that an alternative plan may be necessary. Just because you run a challenge study, it does not guarantee that the formulation or process will work as hoped. What will you do in the event that the product fails?
Guidelines to Consult for Challenge Studies
As noted above, it is important to gather information that will be needed by the expert food microbiologist who will evaluate the risks, consult published information, run predictive models, and design and conduct the study. NACMCF has published recommendations on conducting inoculated pack studies.19 Addressing questions 1–3 outlined in Appendices E–J of the NACMCF document will streamline the preparation process.6
For a more immersive, hands-on experience, participate in the International Association for Food Protection (IAFP) Workshop on Challenge Studies, held annually each spring. The class features lectures, discussion, and breakout sessions where small groups work through the steps on designing, choosing appropriate pathogens for testing, interpreting results, and managing pitfalls that may be encountered.
Health Canada provides specific recommendations for conducting Listeria36 and C. botulinum37 challenge studies. New Zealand offers guidance in shelf life testing that are useful in determining "use by" dates for food safety.38 While the emphasis of the above guidance documents is growth inhibition, other recommendations focus on validating lethality,39 such as for cold-filled hold challenge studies for acidified food products,40 or for validating consumer cooking instructions41 or low-moisture foods.12
Lastly, develop a network of expert food microbiologists you can call on to help you as you navigate through this "challenging" process. They will assist you in ensuring that the work completed is designed to protect public health and your brand.
Acknowledgment
The authors thank Janet Riley of Janet Riley Strategies, Andrew Clarke of Loblaw Companies Ltd., and Mark Beaumont of Danone for their insights shared at the “Food Safety Culture and Communication” session at the 2024 Food Safety Summit.
References
- Schuman, J.D. and B.W. Sheldon. "Thermal Resistance of Salmonella spp. and Listeria monocytogenes in Liquid Egg Yolk and Egg White." Journal of Food Protection 60, no. 6 (1997): 634–638.
- Engstrom, S.K., C. Cheng, D. Seman, and K.A. Glass. “Growth of Listeria monocytogenes in a High-Moisture Cheese on the Basis of pH, Moisture, and Acid Type.” Journal of Food Protection 83 (2020): 1335–1344. http://doi.org/10.4315/jfp-20-069.
- FoodRisk.org. "EcoSure 2007 Cold Temperature Database 2008." January 1, 2007. https://www.foodrisk.org/resources/display/21.
- Weyker, R.E., et al. "Controlling Listeria monocytogenes and Leuconostoc mesenteroides in Uncured Deli-Style Turkey Breast Using a Clean-Label Antimicrobial." Journal of Food Science 81, no. 3 (2016): M672–M683.
- U.S. Food and Drug Administration (FDA). "21 CFR Part 114—Acidified Foods." Code of Federal Regulations. https://www.ecfr.gov/current/title-21/chapter-I/subchapter-B/part-114.
- National Advisory Committee on Microbiological Criteria for Foods (NACMCF). "Parameters for Determining Inoculated Pack/Challenge Study Protocols." Journal of Food Protection 73, no. 1 (2010): 140–202. https://www.sciencedirect.com/science/article/pii/S0362028X22115759.
- Institute for Food Technologists (IFT). "Evaluation and Definition of Potentially Hazardous Foods." Comprehensive Reviews in Food Science and Food Safety 2, Supplement (2003): 1–109.
- ComBase. "ComBase Predictor." 2024. https://combase.errc.ars.usda.gov/default.aspx.
- U.S. Department of Agriculture, Agricultural Research Service (USDA-ARS). "Pathogen Modeling Program (PMP) Online." https://pmp.errc.ars.usda.gov/PMPOnline.aspx.
- DTU National Food Institute. "Food Spoilage and Safety Predictor (FSSP) 4.0." July 2014. http://fssp.food.dtu.dk/.
- Danish Technological Institute (DTI). "DMRI Predict." 2025. https://apps.teknologisk.dk/dmri-predict/.
- Cheng, T., et al. "Methods to Obtain Thermal Inactivation Data for Pathogen Control in Low-Moisture Foods." Trends in Food Science and Technology 112 (2021): 174–187.
- Wang, X. and J. Zhou. "Response of Foodborne Pathogens to Thermal Processing." In Stress Responses of Foodborne Pathogens. T. Ding, X. Liao, and J. Feng, Eds. Springer International Publishing, 2022.
- North American Meat Institute Foundation. "Process Lethality Spreadsheet." 2015. https://meatfoundation.org/IndustryResources/process-lethality-spreadsheet.
- FDA. Draft Guidance for Industry: Hazard Analysis and Risk‐Based Preventive Controls for Human Food. 2018. https://www.fda.gov/media/99581/download.
- International Commission on Microbiological Specifications for Foods. Microorganisms in Foods 5: Characteristics of Microbial Pathogens. Springer Science & Business Media, 1996.
- FDA. Food Code 2022. January 18, 2023 Version. https://www.fda.gov/media/164194/download?at.
- Buchanan, R.L. and S.G. Edelson. "Culturing Enterohemorrhagic Escherichia coli in the Presence and Absence of Glucose as a Simple Means of Evaluating the Acid Tolerance of Stationary-Phase Cells." Applied and Environmental Microbiology 62, no. 11 (1996): 4009–4013.
- Song, W.-J. "Effect of Culture Method on Storage, Plasma, and Dry Heat Treatment Resistance of Salmonella enterica Serovar Typhimurium on Black Pepper." Letters in Applied Microbiology 76, no. 1 (2022).
- Uesugi, A., M. Danyluk, and L. Harris. "Survival of Salmonella Enteritidis Phage Type 30 on Inoculated Almonds Stored at –20, 4, 23, and 35 Degrees C." Journal of Food Protection 69, no. 8 (2006): 1851–1857.
- Enache, E., et al. "Development of a Dry Inoculation Method for Thermal Challenge Studies in Low-Moisture Foods by Using Talc as a Carrier for Salmonella and a Surrogate (Enterococcus faecium)." Journal of Food Protection 78, no. 6 (2015): 1106–1112.
- Gurtler, J.B., et al. "Selection of Surrogate Bacteria in Place of E. coli O157: H7 and Salmonella Typhimurium for Pulsed Electric Field Treatment of Orange Juice. International Journal of Food Microbiology 139, no. 1–2 (2010): 1–8.
- Codex Alimentarius Commission. Guidelines on the Application of General Principals of Food Hygiene on the Control of Listeria monocytogenes in Foods. 2007. https://www.fao.org/fao-who-codexalimentarius/sh-proxy/en/?lnk=1&url=https%253A%252F%252Fworkspace.fao.org%252Fsites%252Fcodex%252FStandards%252FCXG%2B61-2007%252FCXG_061e.pdf.
- Health Canada. "Validation of Ready-to-Eat Foods for Changing the Classification of a Category 1 Into a Category 2A or 2B Food in Relation to Health Canada's Policy on Listeria monocytogenes in Ready-to-Eat Foods (2011)." November 29, 2012. https://www.canada.ca/content/dam/hc-sc/migration/hc-sc/fn-an/alt_formats/pdf/legislation/pol/listeria_monocytogenes-validation-eng.pdf.
- New Zealand Ministry for Primary Industries. Guidance for the Control of Listeria monocytogenes in Ready-to-Eat Foods Part 1: Listeria Management and Glossary. February 13, 2017. https://www.mpi.govt.nz/dmsdocument/16300-Guidance-for-the-Control-of-Listeria-monocytogenes-in-Ready-to-eat-Foods-Part-1-Listeria-Management-and-Glossary.
- U.S. Department of Agriculture Food Safety and Inspection Service (USDA-FSIS). FSIS Cooking Guideline for Meat and Poultry Products: Revised Appendix A. Document ID: FSIS-GD-2021-14. December 2021. https://www.fsis.usda.gov/sites/default/files/media_file/2021-12/Appendix-A.pdf.
- USDA-FSIS. FSIS Directive 711.1, Revision 2: Verification Procedures for Lethality and Stabilization. August 14, 2017. https://www.fsis.usda.gov/sites/default/files/media_file/2020-07/7111.1.pdf.
- USDA-FSIS. FSIS Ready-to-Eat Fermented, Salt-Cured, and Dried Products Guideline. Document ID: FSIS-GD-2023-0002. 2023. May 5, 2023. https://www.fsis.usda.gov/sites/default/files/media_file/documents/FSIS-GD-2023-0002.pdf.
- USDA-FSIS. FSIS Stabilization Guideline for Meat and Poultry Products, Revised Appendix B. Document ID: FSIS-GD-2021-0013. December 2021 .https://www.fsis.usda.gov/sites/default/files/media_file/2021-12/Appendix-B.pdf.
- USDA-FSIS. FSIS Compliance Guideline: Controlling Listeria monocytogenes in Post-Lethality Exposed Ready-to-Eat Meat and Poultry Products. January 2014. https://www.fsis.usda.gov/sites/default/files/import/Controlling-Lm-RTE-Guideline.pdf.
- USDA-FSIS. FSIS Food Safety Guideline for Egg Products. September 9, 2020. https://www.fsis.usda.gov/sites/default/files/media_file/2021-01/food-safety-guideline-egg-products.pdf.
- FDA Center for Food Safety and Applied Nutrition (CFSAN). Guidance for Industry Juice HACCP Hazards and Controls Guidance. March 2004. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-juice-hazard-analysis-critical-control-point-hazards-and-controls-guidance-first.
- California Almonds. "Validation Guidelines." April 13, 2007. https://www.almonds.com/tools-and-resources/food-safety-and-quality/pasteurization/validation-guidelines.
- FDA. Bacteriological Analytical Manual. 8th Edition, Revision A. 1998. https://www.fda.gov/food/laboratory-methods-food/bacteriological-analytical-manual-bam.
- USDA-FSIS. Microbiology Laboratory Guidebook. March 14, 2025. https://www.fsis.usda.gov/news-events/publications/microbiology-laboratory-guidebook.
- Health Canada. Food Directorate, Health Products and Food Branch, Bureau of Microbial Hazards. "Listeria monocytogenes Challenge Testing of Refrigerated Ready-to-Eat Foods." 2012.
- Health Canada. "Clostridium botulinum Challenge Testing of Ready-to-Eat Foods." Version 1. November 24, 2010. https://www.canada.ca/en/health-canada/services/food-nutrition/legislation-guidelines/policies/clostridium-botulinum-challenge-testing-ready-foods-2010.html.
- New South Wales Department of Primary Industries, Food Authority. "Shelf-Life Testing: Use-By Dates for Food Safety." February 2010. https://www.foodauthority.nsw.gov.au/sites/default/files/_Documents/scienceandtechnical/shelf_life_testing.pdf.
- Ceylan, E., et al. "Guidance on Validation of Lethal Control Measures for Foodborne Pathogens in Foods." Comprehensive Reviews in Food Science and Food Safety 20, no. 3 (2021): 2825–2881.
- Breidt, F., E.L. Andress, and B. Ingham. "Recommendations for Designing and Conducting Cold-Fill Hold Challenge Studies for Acidified Food Products." Journal of Food Protection 38, no. 5 (2018): 322–328. https://www.foodprotection.org/members/fpt-archive-articles/18-09-recommendations-for-designing-and-conducting-cold-fill-hold-challenge-studies-for-acidified-fo/.
- Scott, V.N., et al. "Guidelines for Listeria Testing of Environmental, Raw Product and Finished Product Samples in Smoked Seafood Processing Facilities." Food Protection Trends 25, no. 1 (2005): 23–34.
Kathleen Glass, Ph.D. is Emeritus Associate Director and Distinguished Scientist for the Food Research Institute (FRI) at the University of Wisconsin–Madison. Prior to her retirement in January 2025, she worked with the food industry and regulatory agencies to evaluate microbial food safety risks, and design and conduct microbial food challenge studies to identify critical control limits for production. She was a four-term member of the National Advisory Committee for the Microbiological Criteria for Foods (NACMCF) that wrote the inoculated pack study guidelines and taught the International Association for Food Protection (IAFP) Challenge study workshop from 2011 until 2023.
Kristin Schill, Ph.D. is a Research Assistant Professor at the University of Wisconsin–Madison's Food Research Institute (FRI) and leads the Applied Food Safety Laboratory. In this capacity, Kristin works directly with the food industry to design food challenge studies on a wide variety of food products and for foodborne pathogens including Clostridium botulinum, Clostridium perfringens, Listeria monocytogenes, Bacillus cereus, Staphylococcus aureus, and other gram-negative pathogens. Prior to joining FRI, Kristin served as a research microbiologist for the Food and Drug Administration. Dr. Schill is a member of the International Association for Food Protection (IAFP) and co-teaches the IAFP Sponsored Microbial Challenge Testing for Foods Workshop. She also serves on the National Advisory Committee for the Microbiological Criteria for Foods (2023–2025).
Cynthia Austin, M.S. is the Lab Manager for the Biosafety Level 2 (BSL-2) meat processing laboratory at the Meat Science and Animal Biologics Discovery (MSABD) Building at the University of Wisconsin–Madison. In her role, she works with meat companies to design and perform microbiological studies to help ensure safe meat products. Prior to joining UW, she worked for nearly 17 years in Oscar Mayer's Corporate Food Safety Department.


