SCROLL
DOWN
USDA FSIS Declares Salmonella an Adulterant in Breaded, Stuffed Raw Chicken Products
Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images
The U.S. Department of Agriculture's Food Safety and Inspection Service (USDA's FSIS) has announced that it will declare Salmonella to be an adulterant in breaded and stuffed raw chicken products. By declaring Salmonella to be an adulterant, FSIS will have the authority to ensure that contaminated products do not enter the market. The action is part of FSIS' broader efforts to reduce Salmonella illnesses associated with poultry.
Breaded and stuffed raw chicken products are of particular concern because they have been linked to 14 foodborne illness outbreaks and 200 illnesses since 1998. Breaded and stuffed raw chicken products are typically found in the freezer section at grocery stores, and may cause confusion as they can appear cooked; FSIS states that efforts to improve labeling for the products has not been effective at reducing consumer illnesses.
FSIS will propose to set the Salmonella limit of breaded and stuffed raw chicken products at 1 CFU/g. If the products exceed the limit, then they will be considered adulterated and be subject to regulatory action. FSIS will also consider public opinion on whether a different standard for adulteration, such as "zero tolerance" or standards based on specific serotypes, would be more appropriate. The notice is expected to publish in the Federal Register in autumn. After the proposal is finalized, FSIS will announce its final implementation plans and the date on which it will begin routine testing for Salmonella in the specified products.
The U.S. Food and Drug Administration (FDA) recently released its Total Diet Study (TDS) Report for Fiscal Years (FY) 2018–2020. The report summarizes the analytical results of continuous testing done by FDA on food that is collected from retail outlets. In FY 2018, FDA implemented a modernized TDS research design that included streamlined analytical methods, a population-based sampling plan, and an updated food list. The modernized approach increased surveillance of the U.S. food supply, with 87 food collections conducted in FY 2018–2020 that resulted in the sampling of 3,276 food, beverage, and water products. Specifically, the TDS report provides the analytical results for 3,241 samples of 305 foods and beverages analyzed for 21 element analytes, as well as 35 samples of bottled water analyzed for 22 element analytes. A subset of 54 samples including rice-containing foods, juices, and wine were further analyzed for three arsenic species.
Toxic Elements Overview
The report states that key toxic elements of concern—lead, arsenic, cadmium, and mercury—were not detected in the majority (68 percent) of analytical results. Highlighted findings include:
- Lead was detected in 15 percent of samples, with the highest level detected reaching 63 parts per billion (ppb)
- Arsenic was detected in 43 percent of samples, with the highest level detected reaching 10,900 ppb
- Of the subset of 54 samples that were further tested for arsenic, the detected levels of inorganic arsenic ranged from 6.1–103 ppb
- Cadmium was detected in 61 percent of samples, with the highest level detected reaching 400 ppb
- Mercury was detected in 8 percent of samples, with the highest level detected reaching 250 ppb.
Foods with FDA Action Levels Overview
The TDS report also underlines the fact that, for the TDS foods that are subject to FDA action levels or standards, all results fell below FDA established levels. This includes apple juice, for which FDA set an action level of 10 ppb earlier in 2022. Apple juice also has an action level of 10 ppb for inorganic arsenic. Additional TDS foods with FDA action levels include chocolate and hard candy with an action level of 100 ppb for lead, and infant rice cereal with an action level of 100 ppb for inorganic arsenic. FDA standards for bottled water are 5 ppb for lead, 10 ppb for arsenic, 5 ppb for cadmium, and 2 ppb for mercury.
The TDS report also states that, in FY 2019, FDA conducted additional sampling of baby foods. Types of baby foods that were tested for TDS include grape juice, puffed snacks, mixed cereals (dry, multi-grain), dry rice cereals, rice cereals prepared with water, and teething biscuits. Of the 384 samples collected for TDS, 65 percent of associated analytical results for lead, arsenic, cadmium, and mercury had no detectable levels. Bottled water intended for infants also did not have any detectable levels of lead, arsenic, cadmium, nor mercury. FDA believes that the results suggest there are no significant regional or seasonal differences in analyte concentrations in foods intended for babies.

Total Diet Study for 2018–2020 Reveals That No Foods Exceed FDA Action Levels
FDA Releases New Food Fraud Webpage
The U.S. Food and Drug Administration (FDA) has released a new website on economically motivated adulteration (EMA), including food fraud. The purpose of the website is to keep businesses and consumers informed on the latest food fraud developments.
The website includes links on how to report food fraud; examples of food adulteration; how food fraud is detected and monitored; enforcement and legal consequences, such as recalls, seizures, and import refusals; guidance documents to assist manufacturers and importers; and a list of import alerts.
EMA occurs when "someone intentionally leaves out, takes out, or substitutes a valuable ingredient or part of a food," according to FDA. EMA also occurs when a substance is added to a food to make it appear better or of greater value.
Food fraud is a common type of EMA that FDA investigates, but EMA also occurs with other products, including animal food and cosmetics. Some types of EMA are also misbranding violations. Estimating how frequently food fraud occurs or its exact economic impact can be challenging because food fraud is designed to avoid detection. Outside estimates by experts have found that food fraud affects 1 percent of the global food industry at a cost of approximately $10–$15 billion per year, although more recent expert estimates peg the cost as high as $40 billion per year.
Food fraud can also lead to major health issues and even death. Some examples include lead poisoning from adulterated spices and allergic reactions to a hidden or substituted ingredient that contains a small amount of just one food allergen.
Click here to visit FDA’s new EMA website.

Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images
USDA's Food Safety and Inspection Service (USDA's FSIS) has published its annual report on FSIS Foodborne Illness Outbreak Investigations for Fiscal Year 2021 (FY 2021). FSIS investigated nine total foodborne illness outbreaks in FY 2021 compared to 16 in FY 2018, 16 in FY 2019, and 12 in FY 2020. Of the nine outbreaks that FSIS investigated in FY 2021, seven were multi-state outbreaks. The nine outbreaks involved approximately 200 illnesses and 60 hospitalizations. Salmonella was associated with three of the nine outbreaks, and another three outbreaks were linked to Shiga toxin-producing Escherichia coli (STEC). A single outbreak was associated with Listeria monocytogenes. The ninth outbreak involved a suspected case of botulism, but neither botulinum nor Clostridium botulinum were detected in the suspect beef product.
The Salmonella outbreaks comprised four serotypes—Hadar, Enteritidis, Typhimurium, and Infantis—and were linked to turkey, chicken, and pork products; serotypes Typhimurium and Infantis were associated with the same outbreak. The L. monocytogenes outbreaks were linked to chicken and multiple products. The STEC outbreaks involved serotypes O157:H7 and O145, and beef was the most common food product linked to illnesses caused by STEC in FY 2021. The outbreak investigations that occurred in FY 2021 led to three recalls and two public health alerts.
In addition to summarizing its foodborne illness outbreak investigation activity in FY 2021, FSIS also highlighted two key after-action reviews (AARs) in the report. The first AAR was conducted for an outbreak of Salmonella Enteritidis that was associated with raw, frozen, breaded, and stuffed chicken products. The AAR's findings prompted FSIS to bring certain concerns regarding labeling, establishments' food safety programs, and Salmonella sampling in establishments to the National Advisory Committee on Meat and Poultry Inspections (NACMPI). FSIS also encourages manufacturers to review FSIS Labeling Policy Guidance for Uncooked, Breaded, and Boneless Poultry Products.
The second AAR was conducted for an outbreak of L. monocytogenes that was associated with ready-to-eat (RTE) chicken. Whole genome sequencing (WGS) provided FSIS investigators with key data in the L. monocytogenes outbreak investigation. FSIS encourages manufacturers of RTE products that are exposed to the environment post-lethality to review the FSIS Compliance Guideline for Controlling L. monocytogenes.

FSIS Summarizes Foodborne Illness Outbreak Investigations for FY 2021
EU Revises Regulations for Ethylene Oxide in Food Additives
The European Commission has recently published revisions to its regulation on the use of ethylene oxide in food additives. The revisions have been made to provide clarity on the allowable levels of ethylene oxide present in food additives, as uncertainty surrounding regulation of the substance has led to enforcement challenges and recall incidents. Ethylene oxide is a chemical substance with many applications, such as the sterilization of raw materials in food production. However, the substance has been identified as a carcinogenic, mutagenic, and reproductive toxin.
Citing Rapid Alert System for Food and Food Network (RASFF) notifications, the European Commission explains that there have been several recent incidents concerning the presence of ethylene oxide in food products from food additives that contain the substances. The difficulty of discerning the source of ethylene oxide that is present in foods—be it through the unlawful sterilization of food additives or any other reason—has made enforcement of the substance in food challenging.
To avoid future enforcement challenges and recall incidents, the European Commission has clarified that the presence of ethylene oxide, irrespective of its origin, is not authorized for all food additives. The Commission has also set a limit of 0.1 mg per kg for ethylene oxide, including 2-chloro-ethanol expressed as ethylene oxide, in certain additives that are treated with the substance.

Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images
The U.S. Food and Drug Administration (FDA) has issued a supplemental notice of proposed rulemaking that extends the compliance dates for pre-harvest agricultural water provisions as outlined in the recent 2021 Agricultural Water Proposed Rule. The proposed rule, if finalized, will require farms to conduct annual, systems-based agricultural water assessments to determine the necessary measures to minimize potential risks associated with pre-harvest agricultural water.
FDA is now proposing extended compliance dates for the proposed pre-harvest requirements and is also providing clarifying information about the enforcement discretion policy for the harvest and post-harvest agricultural water requirements. The supplemental rulemaking proposes to establish the following compliance dates for the pre-harvest agricultural water requirements for covered produce other than sprouts:
- Two years and nine months after the effective date of a final rule for very small businesses
- One year and nine months after the effective date of a final rule for small businesses
- Nine months after the effective date of a final rule for all other businesses.
The 2021 pre-harvest agricultural water proposed rule did not set forth substantive changes to the harvest and post-harvest agricultural water requirements in the Produce Safety Rule; however, FDA recognizes that prior to the proposal, stakeholders did not have clarity on whether FDA might propose changes to the harvest and post-harvest agricultural water requirements. The agency intends to continue enforcement discretion for the harvest and post-harvest agricultural water requirements of the Produce Safety Rule until the following dates:
- January 26, 2025 for very small businesses
- January 26, 2024 for small businesses
- January 26, 2023 for all other businesses.
For the first year of compliance, FDA intends to work closely with state, regulatory, and industry partners to advance training, technical assistance, educational visits, and on-farm readiness reviews to prepare growers and state regulators for implementing the provisions prior to initiating routine inspections to verify compliance.
For more information on the Proposed Agricultural Water Rule, please see our IAFP interview with Kruti Ravaliya, M.S., Consumer Safety Officer in the Division of Produce Safety at FDA's CFSAN, as part of Food Safety Matters podcast Episode 125.

FDA Extends Deadlines for Agricultural Water Proposed Rule
Economic Impact of Biofilms on Food Industry
A study published in Biofilms and Microbiomes has approximated the economic impact of biofilms on the food industry. The study also describes the existing scientific and technological challenges that must be overcome to progress in the areas of biofilm research and innovation. The study is based on a market analysis and exploratory workshops that were conducted by the UK's National Biofilms Innovation Center (NBIC). According to the study, biofilms cause an estimated $324 billion impact on the global agrifood sector, annually. For context, the world's agricultural activity is valued at approximately $3,700 billion, and the annual economic impact of biofilms across all sectors is estimated at $5,000 billion.
The study states that, at present, the main method for controlling biofilms in the food sector is through the use of sanitation and hygiene practices in food processing facilities. Sanitation and hygiene are effective when properly executed, although they can be expensive to implement and are subject to occasional failure. Additionally, although the use of antibiotics in animal feed was once a popular method for mitigating microbial hazards at the agricultural level, the practice is falling out of favor due to the growing threat of antimicrobial resistance (AMR).
When measures for combatting microbial hazards and biofilms fail, there can be a significant impact on human health and a country's financial resources. The study provides an example of a UK foodborne illness outbreak that originated from produce contaminated by Listeria monocytogenes, which cost the manufacturer €30 million ($35.1 million) in recall, transport, and destruction costs. The study also notes that, in the U.S., foodborne pathogens cause approximately 128,000 hospitalizations each year, resulting in an economic impact of $78 billion, annually.
NBIC's exploratory workshops fostered knowledge-sharing between academics and industry representatives that revealed many scientific and technological challenges that prevent innovation in controlling biofilms. The study lists some of the key areas in which scientific knowledge and technological advancement are necessary, including:
- Understanding the interaction between biofilms and various surfaces
- Knowledge of the matrixome—a contributing factor to certain physiochemical attributes of biofilms—to design more effective interventions
- Rapid early detection systems for real-world applications
- Improved biofilm analysis methods to deepen understanding of biofilm structure and function
- Managing biofilms in natural environments
- Understanding the mode of action of antimicrobials and antibiotics, as well as antimicrobial and antibiotic interactions with biofilms
- Improved models and standards for biofilm evaluation
- Augmenting biofilm repositories and biobanks.
Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images
FDA has released its annual Pesticide Residue Monitoring Program Report for Fiscal Year 2020 (FY 2020), which reveals that the levels of pesticide residues measured by FDA in the U.S. food supply are generally in compliance with the U.S. Environmental Protection Agency's (EPA's) pesticide tolerances. The report summarizes FDA's findings from its monitoring of pesticide residues in human and animal foods in FY 2020.
The report is based on data that was collected from October 1, 2019 through September 30, 2020, during which time FDA tested for approximately 750 different pesticides and selected industrial compounds on 2,078 human food samples (316 domestic and 1,762 imported samples) in its regulatory monitoring program. Agency staff collected domestic human food samples from 35 states and imported human food samples from 79 countries. FDA states that the number of pesticides and industrial compounds analyzed in FY 2020 decreased slightly compared to FY 2019, as compounds that are obsolete or are rarely detected were removed from the agency's scope.
In FY 2020, FDA found that 96.8 percent of domestic and 88.4 percent of imported human foods were compliant with EPA pesticide tolerances. No pesticide residues were found in 40.8 percent of domestic samples and 48.4 percent of imported samples. Among the human food commodity groups, the violation rate in each group was higher for import samples. FDA also analyzed 102 animal food samples (40 domestic and 62 imported samples) for pesticides. The agency found that 100 percent of domestic and 96.8 percent of import animal food samples were compliant with federal standards. No pesticide residues were found in 30 percent of domestic and 48.4 percent of imported animal food samples.

FDA Releases Pesticide Residue Report for FY 2020

Dairy Management Inc. has hired Lori Captain as Executive Vice President of Global Sustainability Strategy, Science, and Industry Affairs.

Michelle Danyluk, Ph.D., assumed the Presidency of the International Association for Food Protection (IAFP) at the conclusion of IAFP 2022.
The American Association of Meat Processors (AAMP) named Darla Kiesel its new President during AAMP's annual convention in 2022.
Standard Meat Company named Tom Allen as the company's new Chief Operating Officer.

Kevin McAdams was appointed President of Perdue Foods and Chief Operating Officer of Perdue Farms.
Goodway Technologies announced that Michael Navaroli has joined the company as Vice President of North American Sales.
TuffWrap Installations Inc., a supplier of dust and debris containment services, has appointed Daniel J. Schmidt as the National Sales Director.
Sokol Custom Food Ingredients has named Antonio Marungo as Director of Culinary Development and Innovation and promoted Max Pombert to Director of Process Development and Commercialization.
PPG packaging coatings announced that Marcio Grossman's role as the PPG General Manager of Latin America South and Packaging Coatings Latin America (LATAM) has expanded to include the U.S. and Canada (USCA) region. Benjamin Jo was promoted to PPG Senior Director of Global Packaging Strategic Accounts. Jenica Eisenbach was named the new PPG global packaging Technical Service Director. Anurag Raj joined as the PPG Customer Sustainability Business Partner, Packaging Coatings.
Arm & Hammer Animal and Food Production has added Greg Schafer as Food Safety Swine Account Manager.
Joe Pezzini, Senior Director of Agricultural Operations for Taylor Farms, has been elected Chair of the Center for Produce Safety (CPS) Board of Directors.

CAPTAIN
MCADAMS
NAVAROLI
AIB International Updates Consolidated Standards for Inspections
The globally recognized AIB International Consolidated Standards for Inspections have been updated to incorporate the latest industry best practices, new regulations, and food safety requirements. The updated consolidated standards were released on July 25, 2022, and inspections against the updated version will begin January 1, 2023.
Major updates include moving written programs to the Adequacy section, dividing the Food Safety Programs standard into two separate standards for Hazard Analysis and Critical Control Points (HACCP) and Hazard Analysis and Risk-Based Preventive Controls (HARPC), expanding the Food Defense standard to provide more guidance for a program that can be applied globally, and creating a separate Regulated Food Defense standard to meet the U.S. Intentional Adulteration requirements.
In 2022, as part of AIB International's commitment to ensuring the integrity of the food supply chain, the company updated five of its Consolidated Standards, including the Consolidated Standards for Prerequisite and Food Safety Programs, Beverage Facilities, Non-Food Contact Packaging Manufacturing Facilities, Food Contact Packaging Manufacturing Facilities, and Food Distribution Centers. The remaining Consolidated Standards will be updated in 2023.

bioMérieux to Conduct Salmonella Quantification in Poultry for FSIS
The U.S. Department of Agriculture's Food Safety and Inspection Service (USDA's FSIS) and bioMérieux have announced the incorporation of bioMérieux's GENE-UP® QUANT Salmonella assay into the methodology used by FSIS regional laboratories. FSIS states that Salmonella quantification is part of the agency's efforts to reduce Salmonella illnesses associated with poultry and to modernize FSIS laboratories' diagnostic capabilities. Using this new system, FSIS will be able to measure the amount of Salmonella present in a regulatory sample, not solely its presence or absence.
Despite the many Salmonella interventions that have been developed over the last two decades, the incidence of Salmonella infections in humans has not improved. Historically, the common method of Salmonella prevalence testing used by the agrifood industry has been a percentage-based approach focusing on the safety of the final product. After evaluating commercially available quantification systems, FSIS determined that GENE-UP® QUANT Salmonella is the most appropriate for sampling raw poultry rinses in its high-throughput laboratory environment. FSIS plans to extend pathogen quantification technology to sample types other than raw poultry rinses in the future.

Intertek Alchemy has published key findings from its seventh Global Food Safety Training Survey. The survey is a comprehensive study and assessment of food safety training practices, drawing on responses from industry professionals representing more than 3,000 food production facilities worldwide. The research reveals what actions and characteristics that companies with robust food safety programs have in common, and what actions the companies with trailing outcomes lack. The report provides an extensive analysis that outlines actionable, data-driven recommendations to help manufacturing companies strengthen their food safety processes.
Intertek Alchemy highlights significant challenges and contradictions of current food safety training practices that were revealed in its survey. For example, 88 percent of companies believe they provide adequate levels of food safety training to drive consistent and appropriate food safety behaviors; however, only 40 percent of their employees follow food safety programs on the manufacturing floor.
Other key findings from the survey include:
- 81 percent of companies understand what it takes to build and sustain a strong food safety culture, but only 22 percent strongly agree that their employees have the authority to take action when food safety is compromised
- 80 percent of companies believe they would be more productive if their employees consistently adhered to their training programs; however, only 19 percent of companies are increasing their budget for food safety training, and only 18 percent plan to acquire new training technology in 2023
- Tailoring training to specific job roles increases the likelihood by 60 percent that a frontline employee will halt production when necessary to prevent a food safety incident
- 78 percent of companies with a mature upskilling program have highly motivated employees, compared to 43 percent for companies without an upskilling program.
Intertek Alchemy Releases Findings from 7th Global Food Safety Training Survey

Safe Foods Begins Expansion Project
Safe Foods Corporation, a division of Packers Sanitation Services Inc. (PSSI), recently began a $14-million project designed to increase the range of products Safe Foods can produce at its Little Rock, Arkansas headquarters. The construction of a new blending plant, including a new reactor and two storage tanks, will double the production capacity for Safe Foods' organic food wash. The project also includes a new, 55,000-square foot packaging facility and warehouse that will allow Safe Foods to manufacture 19 additional product lines for PSSI that will be used to protect consumers from foodborne illness. Plans also include the renovation of a rail spur and new unloading stations.

AEMTEK Expands Services with New Location
AEMTEK, a food safety laboratory and analytical services provider, has opened a new location in Modesto, California. This facility will bring food safety testing closer to current and future clients in California's Central Valley. The facility features state-of-the-art equipment to provide companies with routine food safety testing, environmental monitoring testing and consultation, and research services including shelf life, challenge, and process validation studies. An experienced team of Ph.D. scientists and microbiologists is excited to partner with food manufacturers of the Central Valley to help them achieve their food safety goals and keep the community safe.


ONLINE & OF NOTE
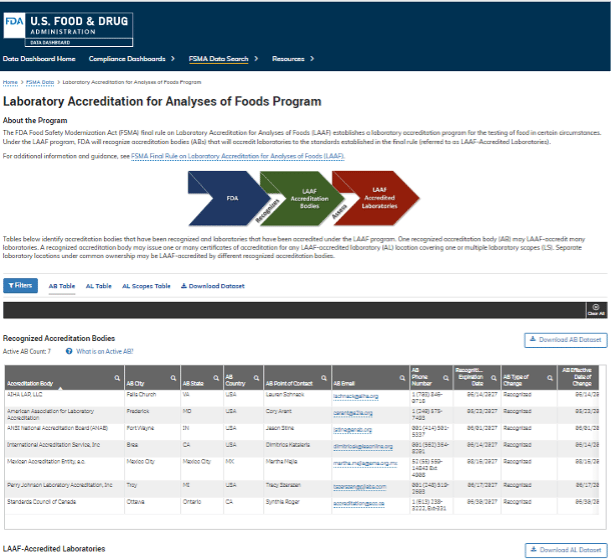
A new Laboratory Accreditation for Analyses of Foods (LAAF) dashboard has been added to the U.S. Food and Drug Administration (FDA) data dashboard to serve as the online public registry listing for information on participants in the LAAF program. The functionality of the LAAF dashboard includes searching; viewing; and the ability to export the lists of recognized accreditation bodies, LAAF-accredited laboratories, and each laboratory's testing scopes. Interested accreditation bodies may apply for recognition under the LAAF program through an electronic portal. Interested laboratories may apply directly to one of the recognized accreditation bodies to seek LAAF accreditation.
The Allergen Bureau has launched its new "Assessing Agricultural Cross-Contact 2022" guide, which aims to assist agrifood businesses in mitigating allergen cross-contamination. In addition to the guide, the Allergen Bureau's Sampling and Testing working group has developed a questionnaire that industry can use to determine the level of risk that may be associated with raw materials, as well as learn how the development of sampling plans can be guided by risk and how facilities can discern the presence and prevalence of allergen cross-contact. The guide, in conjunction with the raw material risk matrix questionnaire, enables businesses to identify risks and take action. The risk rating can be used to inform sampling approaches for verification activities to understand the presence and prevalence of cross-contact. The new resource can also help the VITAL (Voluntary Incidental Trace Allergen Labeling Program) risk assessment process.