SCROLL
DOWN
The U.S. Food and Drug Administration (FDA) has announced the selection of James (Jim) Jones, M.S. to serve as the first Deputy Commissioner for Human Foods, who will lead the charge in setting and advancing priorities for a proposed unified Human Foods Program (HFP). Mr. Jones was scheduled to begin at the FDA on September 24, 2023.
Program areas that the Deputy Commissioner for Human Foods will oversee include food safety, chemical safety, and innovative food products, such as new agricultural technologies, to bolster the resilience of the U.S. food supply in the face of climate change and globalization. His scope will also include nutrition to help reduce diet-related diseases and improve health equity. A recently released graphic video provides a high-level overview of FDA's proposed vision for the unified HFP.
In the role of Deputy Commissioner for Human Foods, Mr. Jones will report directly to FDA Commissioner Robert M. Califf, M.D. He will exercise decision-making authority over all HFP entities when the reorganization is in effect, including related Office of Regulatory Affairs (ORA) activities. He will provide executive leadership over the entire program as well as over resource allocation, risk-prioritization strategy, policy, and major response activities involving human foods. The leadership for Center for Food Safety and Applied Nutrition (CFSAN) and Office of Food Policy and Response (OFPR) will report to Mr. Jones until the proposed HFP reorganization is implemented.
For more than 30 years, Jones has held various positions in the U.S. Environmental Protection Agency (EPA), stakeholder community, and private industry, where he has managed teams and provided strategic planning and thought leadership around issues related to chemical safety and sustainability in the environment. At EPA, he was a principal architect of the 2016 overhaul of the Toxic Substances Control Act, the first update of that statute in more than 40 years. He was also responsible for decision-making related to the regulation of pesticides and commercial chemicals. Additionally, Mr. Jones was an integral member of the Reagan-Udall Foundation's Independent Expert Panel for Foods, which submitted a report on the operational evaluation of the FDA's Human Foods Program to the agency in December 2022, making him knowledgeable of the agency's challenges and opportunities.

FDA Appoints Jim Jones as First Deputy Commissioner for Human Foods
FAO/WHO Expert Meeting Will Inform Updates to Codex Guidance on Foodborne Viruses
Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images
The Joint Food and Agriculture Organization of the United Nations (FAO)/World Health Organization (WHO) Expert Meeting on Microbiological Risk Assessment (JEMRA) held a meeting on September 18–22, 2023 in Rome, Italy to discuss food attribution, analytical methods, and indicators of viruses in foods.
The JEMRA meeting was requested by the Codex Alimentarius Committee on Food Hygiene (CCFH) to provide scientific advice to inform the review of Codex Guidelines on the Application of General Principles of Food Hygiene to The Control of Viruses in Food (CXG 79-2012). The purpose of the guidelines is to provide direction on how to prevent or minimize the presence of human enteric viruses in foods, especially hepatitis A and norovirus.
To support the update of the guidance, CCFH has requested that JEMRA provide scientific advice on the following areas, giving priority to items 1, 3, and 4:
- An up-to-date review of the foodborne viruses and relevant food commodities of highest public health concern
- A review of the scientific evidence on prevention and intervention measures and the efficacy of interventions in the food continuum
- A review of the analytical methods for relevant enteric viruses in food commodities
- A review of scientific evidence on the potential utility of viral indicators or other indicators of contamination
- A review of the various risk assessment models with a view towards constructing more applicable models for wide use among member countries, including a simplified risk calculator.
Using whole genome sequencing (WGS), the U.S. Centers for Disease Control and Prevention (CDC) has identified a reoccurring, emerging, and persistent (REP) strain of Escherichia coli O157:H7 that was implicated in a significant foodborne illness outbreak linked to romaine lettuce in 2019. The strain, REPEXH02, is believed to have emerged in late 2015 and caused multiple outbreaks from 2016–2019.
In the article classifying REPEXH02 as a REP strain, CDC concluded that 58 percent of recent E. coli infections can be attributed to vegetable row crops, mostly leafy greens. The strain of E. coli in question was found to be the cause of an outbreak in 2019 associated with romaine lettuce grown in the Salinas Valley region of California, causing 167 cases of illness across 27 states that resulted in 85 hospitalizations. Additionally, a late 2020 outbreak also linked to leafy greens sickened 40 people in 19 states, resulting in 20 hospitalizations and four cases of hemolytic uremic syndrome (HUS).
Shortly after the late 2020 outbreak, acknowledging the fact that foodborne illness outbreaks associated with leafy greens grown in the California Central Coast region (encompassing Salinas Valley) had occurred every fall since 2017, the FDA launched an investigation into the 2020 outbreak and used WGS to link the implicated strain with prior outbreaks back to 2017. FDA named the E. coli strain REPEXH02 as a "reasonably foreseeable hazard."
In their recent article, CDC stated that cattle are the main reservoir for E. coli O157:H7, which is entering leafy greens irrigation water due to cattle fecal matter contamination being carried by floodwaters. Further genomic characterization for this REP strain is required to explain what causes its emergence and persistence in different environments.

CDC Identifies Significant REP Strain of E. coli Causing Leafy Greens Outbreaks
FDA Releases New Food Fraud Webpage
The U.S. Food and Drug Administration (FDA) has released a new website on economically motivated adulteration (EMA), including food fraud. The purpose of the website is to keep businesses and consumers informed on the latest food fraud developments.
The website includes links on how to report food fraud; examples of food adulteration; how food fraud is detected and monitored; enforcement and legal consequences, such as recalls, seizures, and import refusals; guidance documents to assist manufacturers and importers; and a list of import alerts.
EMA occurs when "someone intentionally leaves out, takes out, or substitutes a valuable ingredient or part of a food," according to FDA. EMA also occurs when a substance is added to a food to make it appear better or of greater value.
Food fraud is a common type of EMA that FDA investigates, but EMA also occurs with other products, including animal food and cosmetics. Some types of EMA are also misbranding violations. Estimating how frequently food fraud occurs or its exact economic impact can be challenging because food fraud is designed to avoid detection. Outside estimates by experts have found that food fraud affects 1 percent of the global food industry at a cost of approximately $10–$15 billion per year, although more recent expert estimates peg the cost as high as $40 billion per year.
Food fraud can also lead to major health issues and even death. Some examples include lead poisoning from adulterated spices and allergic reactions to a hidden or substituted ingredient that contains a small amount of just one food allergen.
Click here to visit FDA’s new EMA website.

Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images
The U.S. EPA has released the first dataset collected under the fifth Unregulated Contaminant Monitoring Rule (UCMR 5), which is intended to provide new information that will improve EPA's understanding of the frequency that 29 per- and polyfluouralkyl substances (PFAS) and lithium are found in U.S. drinking water systems, and at what levels. The monitoring data on PFAS and lithium will help the EPA make determinations about future actions to protect public health under the Safe Drinking Water Act.
The data collected under UCMR 5 will ensure science-based decision-making and help EPA better understand national-level exposure to 29 PFAS and lithium, and whether they disproportionately impact communities with environmental justice concerns. The initial data release represents approximately 7 percent of the total results that EPA expects to receive over the next three years. The agency will update results quarterly and share them with the public in EPA's National Contaminant Occurrence Database (NCOD) until completion of data reporting in 2026.
In March 2023, EPA proposed standards to limit certain PFAS in drinking water. The proposal, if finalized, would allow public water systems to use results from UCMR 5 to meet the rule's initial monitoring requirements and to inform communities of actions that may need to be taken. In the interim period before the PFAS drinking water standard is final, EPA has established Health Advisories (HAs) for four PFAS included in the UCMR 5.
Based on the limited initial set of data, EPA concluded:
- PFOA and PFOS are two of the most widely studied PFAS. One or each of these two PFAS was measured at or above EPA's minimum reporting level (MRL), and therefore above EPA's HA levels, in the first sampling event for 7.8–8.5 percent of public water systems (PWSs) with results to date.
- The other two PFAS with EPA HA levels are HFPO-DA (also known as "GenX chemicals") and PFBS. HFPO-DA was measured above the HA level in 1 of 2,002 PWSs. PFBS was not found above the HA level.
- HA levels have not been established for the other 25 PFAS that are part of UCMR 5. Nine of these 25 PFAS were measured at or above their respective MRL in 1–207 of approximately 2,000 PWSs. For the other 16 PFAS, no PWSs have reported results at or above their respective MRLs.
- EPA has not published a HA level for lithium but has calculated a Health Reference Level (HRL) for screening purposes. To date, 22 percent of PWSs have reported lithium results above the screening HRL.
EPA is deploying $9 billion to invest in communities with drinking water impacted by PFAS and other emerging contaminants, including $4 billion via the Drinking Water State Revolving Fund (DWSRF) and $5 billion through EPA's "Emerging Contaminants in Small or Disadvantaged Communities" grant program. The funds will help communities make investments in solutions to remove PFAS from drinking water.

EPA Releases First Dataset for Project Monitoring PFAS, Lithium in U.S. Drinking Water
According to the recently released 2020–2021 Activity Report for the International Food Safety Authorities Network (INFOSAN), the INFOSAN Secretariat responded to 375 international food safety events, which is nearly double the number of incidents in 2018–2019 (162) and the highest number since the network was established in 2004. INFOSAN was established by the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization (WHO) for the purpose of connecting authorities in different countries to strengthen national and international food safety systems.
The role of the INFOSAN Secretariat during food safety events is to facilitate communication among FAO/WHO Member States and encourage information-sharing about essential details of implicated products and their distribution, helping to identify the sources of foodborne illness outbreaks in a timely manner. The increase in responses by the INFOSAN Secretariat to food safety events in 2020–2021 could be attributed to the increased awareness of food safety risks, capacity-building activities delivered by the INFOSAN Secretariat, increased reporting of food safety issues, webinars and workshops with FAO and WHO Member States, increased capacity of the INFOSAN Secretariat, and stronger collaboration with key partners. Additionally, the COVID-19 pandemic, which was at its height during the reporting period, provided motivation for Member States to strengthen food safety systems, including international and cross-sector collaboration for emergency response and risk communication, to ensure the safety of food distributed internationally.
Like in 2018–2019, biological hazards were the most significant cause of food safety events in 2020–2021, accounting for 57 percent (212 of 375) of incidents. Undeclared allergen, physical, and chemical hazards followed, accounting for 17 percent, 13 percent, and 11 percent of food safety events, respectively. The remaining incidents were caused by an unknown hazard.
Salmonella was the most common cause of biological food safety events, responsible for 41 percent of incidents, followed by Listeria monocytogenes (25 percent), Escherichia coli (8 percent), and Clostridium (7 percent). The most implicated food categories were fish and other seafood (17 percent), followed by milk and dairy products (10 percent), meat and meat products (10 percent), and snacks and desserts (9 percent). Noting that multiple regions are often involved in the same events, the majority of the 375 events involved Member States in the European Region (68 percent), followed by the Western Pacific Region (27 percent), the Region of the Americas (27 percent), the African Region (23 percent), the Eastern Mediterranean Region (16 percent), and the Southeast Asia Region (9 percent).
The 2020–2021 Activity Report highlights two major outbreaks that the INFOSAN Secretariat was involved in linked to the consumption of enoki mushrooms from the Republic of Korea in 2020 and Galia melons from Honduras in 2021. During these incidents, INFOSAN facilitated international collaboration between the affected countries to identify the sources of the outbreaks, share investigation information, and implement risk mitigation measures.

INFOSAN Responded to Most Ever Food Safety Incidents in 2020–2021
Scientists from the National Research Council of Canada (NRC), alongside collaborators from the U.S. and Norway, have discovered the algal source of ciguatoxin in Caribbean waters. Approximately 500,000 people around the world are affected by ciguatera poisoning each year, caused by the consumption of fish species like red snapper that feed on ciguatoxin-producing algae. The groundbreaking finding will make it possible to develop methods to understand the distribution of ciguatoxins across the food web, and to establish standards to help food safety laboratories monitor and manage the risk of ciguatera poisoning.
Ciguatoxins have historically posed a food safety risk in fish sourced from the Caribbean Sea, the Indian Ocean, and the Pacific Ocean; however, because of the effects of climate change in recent years, ciguatoxin has also become a hazard in the waters surrounding the Canary Islands, the eastern Mediterranean Sea, and the western Gulf of Mexico. Although the algal source of ciguatoxins in the Pacific was identified many years ago, the Caribbean source remained unknown, despite nearly 30 years of research.
In 2023, the algal source of ciguatoxin was finally identified. The discovery was the result of a concerted effort launched in 2018 and led by NRC's Biotoxin Metrology group. Collaborators on the project included experts from the University of South Alabama, the University of Texas at Austin, the University of the U.S. Virgin Islands, and the Norwegian Veterinary Institute. After NRC researchers identified the novel algal ciguatoxin, the U.S. and Norwegian collaborators helped demonstrate metabolism in fish species associated with ciguatera poisoning.
The U.S. scientists collected algae samples from coral reefs in the Caribbean, established cultures to grow algae, and then conducted toxicity screenings. After toxic strains were received from the U.S., NRC researchers used high-resolution mass spectrometry and innovative chemistry techniques to characterize the algae's chemical profile, enabling them to determine toxin structure and identify the unknown ciguatoxin. Finally, Norwegian researchers conducted enzyme incubation experiments to confirm that the algal toxin transforms into the toxin found in fish that causes ciguatera poisoning.
NRC will continue working with other teams to develop next-generation metrological tools for managing ciguatoxins. The group will also work with collaborators to create certified reference materials that global testing and research labs can use to measure ciguatoxins.
After 30 Years of Research, Scientists Discover Source of Ciguatoxin in Caribbean

Ocean Mist Farms appointed Kate Burr as Director of Food Safety and Quality Assurance.
Golden State Foods promoted Brian Dick to President and Chief Executive Officer; John Page to Executive Vice President and Chief Administrative Officer; Brad Tingey to Corporate Senior Vice President and Chief Financial Officer; and Stephen Wetterau to Corporate Senior Vice President of Strategy, Technology, and Innovation.
SCS Global Services appointed Denise Webster as Vice President of Food Safety.
Frank Yiannas was recently appointed to the Board of Directors of iFoodDS. He also joined Revol Greens as an Advisor for food safety, and Wiliot as a Strategic Advisor for his traceability expertise.
Tom Eickman was named President of American Association of Meat Processors (AAMP) for 2023–2024. Scott Filbrandt was appointed Treasurer. Shane Flowers was named First Vice President, Louis Fantasma was appointed Second Vice President, and Joel Reck was named Third Vice President.
Oklahoma Secretary of Agriculture Blayne Arthur was appointed as the 2023-2024 President of the National Association of State Departments of Agriculture (NASDA). Also elected to NASDA's Board of Directors were Arkansas Secretary of Agriculture Wes Ward (Vice President) and Maine Commissioner of Agriculture Amanda Beal (Second Vice President).
Bel Group promoted Béatrice de Noray to Chief Growth Officer.
CRB hired Carl Williams as its new Vice President of Environmental Health and Safety.
Hydrite announced Steve Tienvieri as its new Senior Lead Microbiologist.
PPG announced Claudia Knotts as the Technical Director of its Americas Packaging Coatings business, and appointed Elzen Kurpejovic as the Technical Director of its EMEA Packaging Coatings business.

BURR

WEBSTER

YIANNAS
Scientists Develop Formulation That Boosts Antimicrobial Properties of Natural Hand Barrier
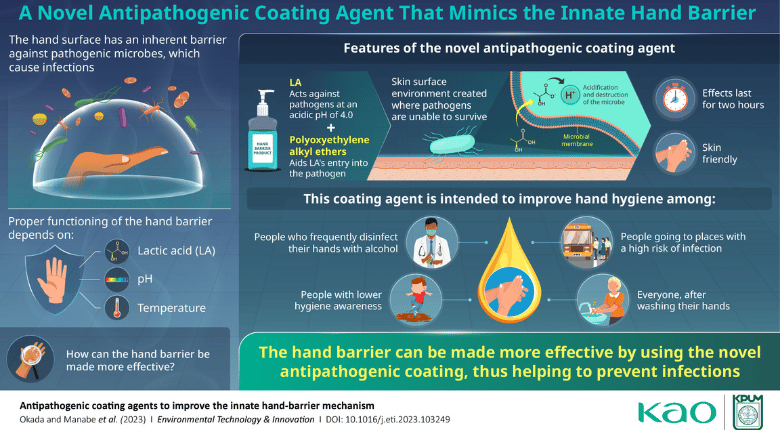
Scientists from Kao Corporation recently discovered the mechanisms by which the natural human hand barrier can fight off pathogens, and have leveraged this information to develop a novel, long-lasting skin coating agent that boosts the antimicrobial properties of the hand barrier. The researchers found lactic acid to be the main active component of the natural hand barrier. Specifically, sweat causes lactate to accumulate on the skin of the hands, where the low surface pH converts lactate into lactic acid. The relatively high temperature of the hands disrupts the membrane of microbes and makes them permeable to lactic acid. Once inside a pathogen, lactic acid transforms back into lactate and releases a proton that lowers the internal pH of the microbe, causing DNA and metabolic damage.
Seeking a practical way to leverage this information to help prevent the spread of pathogens, scientists from Kao Corporation joined forces with experts at the Kyoto Prefectural University of Medicine to develop a coating agent that can greatly boost the antimicrobial properties of the natural hand barrier. This development opens the doors for new hand hygiene solutions that could potentially help food handlers prevent cross-contamination.
MenuTrinfo's "Certified Free From" (CFF) seal can support brands' food safety and boost sales by indicating third-party validation of allergen-free claims on a product label. CFF assures allergen-free label claims, having been accredited by the American National Standards Institute National Accreditation Board (ANSI ANAB) for meeting International Organization for Standardization (ISO) 17065 standards for certifying bodies.
MenuTrinfo's "Certified Free From" Seal Assures Allergen-Free Label Claims

Hygiena® recently announced that the U.S. Patent and Trademark Office has granted patent number 11,634,782, U.S. Patent Application No. 16/549,059, and an official registered trademark to protect its BAX® System SalQuant®. The patent has been effective since April 23, 2023. Hygiena's patented SalQuant, which was developed to meet increased demand for Salmonella quantification in One Health Diagnostics™ applications, serves as an alternative to traditional, time-consuming methods. Using the multipurpose tool, processors can quantify Salmonella from the farm to final product to improve and verify sanitation and antimicrobial intervention processes, meeting more stringent regulatory and quality standards while protecting consumers by knowing the risk a positive sample could pose.

Hygiena Announces New U.S. Patent for SalQuant Salmonella Quantification
Duravant's newly formed Food Sorting and Handling Solutions group includes operating companies Key Technology, Multiscan Technologies, PPM Technologies, and WECO. The group provides a range of optical sorters, specialized conveyors, and advanced turnkey processing line solutions. Examples of technologies offered by Duravant's Food Sorting and Handling Solutions group include Key Technology's new COMPASS® food optical sorter and its reversible Zephyr™ horizontal-motion conveyor, WECO's integrated Sortivator fresh-pack line for blueberries, Multiscan's vision technology for sorting, and PPM Technologies' Mini VF electromagnetic vibratory motion conveyor for product handling.

Duravant's Food Sorting and Handling Solutions Group Features Range of Technologies
The National Institute of Oilseed Products (NIOP) has formed a Product Integrity Committee (PIC) in response to increasing incidents of food fraud within the oilseed industry worldwide. The committee will closely investigate the issue of food fraud, collect data from member companies, and educate local, state, and federal leaders responsible for overseeing the health, safety, and labeling of packaged goods on why the critical issue must be addressed. NIOP members have also begun quality testing oil shipments at many of its members' facilities, which will provide additional data on the prevalence of fraud within the industry.
Oilseed fraud typically occurs by short weighting products or substituting high-quality oils with inferior oils to increase profit margins. Fraudulent oils may have an inferior nutritional quality and value for consumers. In establishing the PIC, NIOP has elected to become a comprehensive resource offering the latest news and information on food fraud within the oilseed industry, and to help its members advocate for meaningful policy enforcement and updates for protecting the integrity of weight and ingredients in packaged products.
NIOP Establishes Product Integrity Committee to Address Food Fraud


ONLINE & OF NOTE
The Food and Agriculture Organization of the United Nations (FAO) is offering an online course through its FAO eLearning Academy on the threat that antimicrobial resistance (AMR) poses to global health, the role of the food and agriculture sector, and the impact of AMR on agrifood systems. The course also describes how FAO is contributing to increased awareness and engagement of all stakeholders in the food and agriculture sector to tackle AMR.
The course was developed by FAO in collaboration with the UK Veterinary Medicines Directorate (VMD), with the support of the UK Center for Environment, Fisheries, and Aquaculture Science (CEFAS), and the UK Animal and Plant Health Agency (APHA). The course takes 2 hours and 50 minutes to complete, is available in English, and covers the following topics:
- General principles of the emergence and spread of AMR in agrifood systems
- Relevance and impacts of AMR in the food and agriculture sector
- The potential role of governments, farmers, animal health professionals, industry, and other key stakeholders in AMR mitigation
- The role and current initiatives of FAO in tackling AMR and the FAO Action Plan on AMR 2021–2025.