
SCROLL
DOWN
The U.S. Centers for Disease Control and Prevention (CDC) recently made major cuts to its Foodborne Diseases Active Surveillance Network (or FoodNet) surveillance program, citing inadequate funding. FoodNet is a joint effort between CDC, the U.S. Food and Drug Administration (FDA), the U.S. Department of Agriculture (USDA), and ten state health departments. According to a CDC spokesperson who talked to NBC News, as of July 1, surveillance under FoodNet has been reduced from eight to two foodborne pathogens and now only covers Shiga toxin-producing Escherichia coli (STEC) and Salmonella.
Before July, FoodNet covered six additional pathogens that are significant to food safety and public health: Campylobacter, Cyclospora, Listeria monocytogenes, Shigella, Vibrio, and Yersinia. According to CDC's latest estimates, which were informed in part by FoodNet data, Campylobacter alone caused 1.9 million cases of foodborne illness in the U.S. in 2019—which beat out both Salmonella and STEC. Additionally, although Listeria monocytogenes typically causes fewer cases than other pathogens, infections caused by this pathogen can be very deadly.
Food safety scientists and medical experts are concerned that the recent cuts to FoodNet may compromise the ability of public health officials to recognize when cases of illness related to a certain pathogen begin to rise, which could hinder foodborne illness outbreak response. An accurate analysis of trends over time may also be impacted.
Although state health departments are no longer required to track data for the six pathogens cut from FoodNet, they can continue to conduct surveillance for those pathogens on their own, if they choose. For example, the Maryland Health Department told NBC News that it would continue reporting for all eight pathogens regardless of changes to FoodNet. On the other hand, the Colorado Department of Public Health and Environment said it would need to scale back surveillance if funding is decreased in fiscal year 2026.
In other trending CDC news, President Trump's pick for CDC Director, Dr. Susan Monarez, who was confirmed by the Senate in July, has been fired from her position due to her disagreements with Secretary Kennedy on vaccine policy and his stance on the role of vaccines in autism. Dr. Monarez initially refuted her dismissal, although the White House leveraged its authority and directly fired her.
Dr. Monarez had also refused to fire three veteran CDC officials—Dr. Debra Houry, Chief Medical Officer and Deputy Director of Programs and Science; Dr. Demetre Daskalakis, Director of the National Center for Immunization and Respiratory Diseases; and Dr. Dan Jernigan, Director of the National Center for Emerging and Zoonotic Infectious Diseases. After Dr. Monarez's dismissal was confirmed, however, Dr. Houry, Dr. Daskalakis, and Dr. Jernigan also announced they would be leaving the agency.
Dr. Monarez was originally nominated by President Trump for the CDC Director position after withdrawing his previous choice, Dr. David Weldon, after it seemed he wouldn't be able to secure the votes in Senate required for his confirmation. Dr. Monarez was seen as a less controversial nominee due to not sharing Dr. Weldon's vaccine skepticism.
The firing of Dr. Monarez and the resignation of veteran top officials comes during an already tumultuous time for CDC. The agency has been on the receiving end of significant workforce and budget cuts since the Trump Administration entered the White House, including the dismissal of more than 2,000 staffers.

CDC Slashes FoodNet Pathogen Surveillance; Top Officials Resign After Director Firing
Reagan-Udall Foundation's 'Roadmap to Produce Safety' Encourages Private Sector-Led Collaboration
Image credit: Dr_Microbe/iStock / Getty Images Plus via Getty Images

The Reagan-Udall Foundation has published a report based on months of stakeholder dialogues, titled, Roadmap to Produce Safety, which illustrates what an enhanced produce food safety system might look like. It also provided recommendations for a private sector-led collaborative to improve produce safety.
The report was compiled based on the Produce Safety Dialogue process, which involved more than 170 experts engaged in eight work groups between September 2024 and April 2025, and was led by a steering committee of representatives from industry and consumer advocacy groups. The project was funded by a $100,000 award from FDA and the U.S. Department of Health and Human Services (HHS).
The importance of fresh produce to a healthy diet is undisputed, but so is the complexity of ensuring the microbial safety of fruits and vegetables due to the lack of a final kill step (e.g., cooking) in their preparation. Additionally, according to the report, the produce safety system is also "fragmented, reactive, and providing too little support to growers—large and small, domestic and foreign—to make produce as safe as reasonably possible." In light of these challenges, the Produce Safety Dialogue aimed to forge a path forward to improve produce safety.
Summarizing the Produce Safety Dialogue, the Roadmap to Produce Safety presents the key opportunities identified by participants to revolutionize how produce safety is fundamentally managed. Participants in the dialogue concluded that "progress requires more than just regulations—it requires leadership from the breadth of the fresh produce supply chain and adjacent sectors," which must include "active investment by produce industry leaders, in addition to government engagement and support," to develop a lasting mechanism for change.
Specifically, this mechanism for change would be a private-sector-led, structured, standalone, sustainably funded stakeholder collaboration (SSC). The report recommends that a subset of Produce Safety Dialogue participants, representative of the entire produce supply chain, should initiate the SSC. The Roadmap to Produce Safety outlines what a successful SSC could look like.
Importantly, the SSC would "provide the leadership and infrastructure, such as project management, to help collaborators develop and maintain trust to then achieve objectives of the Strategic Roadmap and have a mechanism for tracking progress against goals." It would need to address deeply rooted issues exposed through the Produce Safety Dialogue, such as a lack of trust and systemic power and economic differentials.
After addressing the issues required to move forward collaboratively, the SSC could work toward improving produce safety through a coordinated set of activities over a multi-year period. The Produce Safety Dialogue identified several strategic opportunities:
- Make the case for and develop a strategy to increase public investment in produce safety. An organized effort inclusive of the broadest possible range of public and private stakeholders should promote the need for and seek greater public investment in produce safety to reduce illness and foster greater consumption for healthy diets.
- Focus on developing and implementing science-based, risk-reducing best practices. Buyers, growers, and other produce safety stakeholders should collaborate with FDA on creating and implementing processes for private sector-led development of best practices that target and reduce significant food safety risks.
- Expand technical assistance and other resources to support industry implementation of best practices. The produce industry, government agencies, academia, and other stakeholders should collaborate to systematically identify the resource needs and technical support requirements for growers and mobilize extension and other resources to meet them.
- Increase incentives for growers to implement best practices. Buyers and regulators should create incentives and remove obstacles to implementation of best practices and reward implementation of key best practices.
After an SSC is established, it should define its priorities for a given timeframe. Although the priorities suggested during the Produce Safety Dialogue (e.g., the issue of agricultural water) can serve as a useful starting point, further scoping is required to agree on a path forward.
Some examples of activities identified during the Produce Safety Dialogue that would benefit produce safety include data-sharing to support a One Health approach to managing fresh produce production and animal agriculture, and to facilitate knowledge transfer based on learnings from outbreaks.
The Roadmap to Produce Safety calls to attention the creativity required in the face of continuously inadequate funding for produce safety activities. "While funding for produce safety activities—research, extension, and implementation—has historically never been commensurate with the public health priority of fruit and vegetable consumption, this chasm is only widening with cuts to federal and state funding," it says. "This reality requires the private sector to rethink how to collaborate with stakeholders, including policymakers, researchers, and extension specialists to improve produce safety."
Regarding the report, FDA said it will actively participate in the SSC efforts and agrees with the recommendation that regulators should not own or lead the coalition. Read the full Roadmap to Produce Safety report here.
The U.S. Food and Drug Administration's Human Foods Program (FDA's HFP) has published a proposed agenda for guidance document work to be advanced in 2025. This work may include the drafting of new guidance documents or proposing revisions to existing guidance.
Priority topics the agency intends to address through completed guidance documents in 2025 include:
- An action level for opiate alkaloids on poppy seeds
- The use of food colorants from natural sources (i.e., fruit and vegetable juices)—an effort that was first alluded to in FDA's April 2025 announcement about working with industry to phase out synthetic food dyes from the U.S. food supply
- Identity and safety information for new dietary ingredient (NDI) notifications.
Additional topics FDA may focus on in this year's guidance document work are:
- Action levels for cadmium in food intended for babies and young children
- Action levels for inorganic arsenic in food intended for babies and young children
- Hazards analysis and risk-based preventive controls for chemical hazards for human foods
- The Food Traceability Final Rule, also known as section 204(d) of the Food Safety Modernization Act (FSMA 204), the compliance date for which was recently extended from January 2026 to July 2028.
The full list of HFP guidance under development can be accessed on FDA's website. The agency may issue additional guidance that is not on the list.

FDA Plans to Issue Guidance on Natural Food Colorants, Other Priority Topics in 2025
Alongside the July opening of the USDA-FSIS Midwestern Food Safety Laboratory in Normandy, Missouri, Secretary Rollins unveiled the agency's new food safety policy, which is intended to reduce instances of foodborne illness through more stringent food safety inspections, enhanced testing procedures, and more rapid outbreak response.
Several points of focus in the plan are reminiscent of failures and vulnerabilities revealed by the deadly Boar's Head listeriosis outbreak of 2024, upon which USDA promised to investigate and take action; for example, USDA's regulatory approach to Listeria monocytogenes, Talmadge-Aiken state cooperative inspection programs, and taking action against noncompliant firms.
The five-part plan focuses on:
1. Microbiological Testing and Inspection Oversight
USDA is making continued enhancements to its Listeria testing method to provide results to industry more quickly and to detect a broader set of Listeria species. Additional results will highlight conditions where Listeria monocytogenes can thrive in facilities producing ready-to-eat (RTE) products and help industry and FSIS identify potential sanitation problems. So far in 2025, FSIS has tested over 23,000 samples for Listeria, a more than 200 percent increase in samples from 2024.
FSIS is also mobilizing its resources to perform more robust, in-person Food Safety Assessments (FSAs), prioritizing RTE meat and poultry establishments. In 2025, the agency completed 440 FSAs, a 52 percent increase from the same period in 2024. These reviews proactively identify and address potential food safety concerns.
2. Equipping FSIS Inspectors with Updated Training and Tools
In 2025, FSIS implemented a new weekly questionnaire for frontline inspectors to collect data on specific Listeria monocytogenes-related risk factors at all RTE establishments. This new tool collects important data to identify developing food safety concerns, allowing FSIS inspectors and their supervisors to take timely action to protect consumers. To date, approximately 53,000 weekly questionnaires with over 840,000 new data points have been collected on these risk factors.
To complement the questionnaire, FSIS continues to enhance its instructions and related training for inspectors to help them recognize and elevate problems with an establishment's food safety system. New instructions aid inspectors in recognizing how to look beyond individual noncompliances and determine when an establishment has systemic problems that should be elevated and addressed. Since January, the agency also updated its Listeria-specific training and administered it to over 5,200 frontline inspection personnel. This training will strengthen inspectors' understanding of the regulatory requirements in FSIS' Listeria Rule and how to verify that establishments have designed and implemented food safety systems that comply with those requirements.
3. Reducing Salmonella Illnesses Attributable to Poultry
Secretary Rollins has charged FSIS to find a more effective and achievable approach to address Salmonella in poultry products after withdrawing the previously proposed Salmonella Framework in April, citing "significant concerns raised by stakeholders about the regulatory burden and costly impacts it would have had on small poultry growers and processors." According to USDA, "the Trump Administration is pursuing a new, common-sense strategy on Salmonella to protect public health while preventing unnecessary regulatory overreach, which will begin by convening listening sessions with key stakeholders to collaborate on best approaches moving forward."
The previously proposed regulatory framework was developed after extensive consideration by the National Advisory Committee on Microbiological Criteria for Foods (NACMCF), an expert scientific advisory committee that was disbanded by the Trump Administration in March, as well as several rounds of stakeholder consultation. The previously proposed framework was supported by consumer protection groups like STOP Foodborne Illness and Consumer Reports. Critics of the framework included poultry industry representatives like the National Chicken Council.
4. Strengthening State Partnerships
States are crucial partners in ensuring a safe and strong food supply and provide a vital service in bringing nutritious, affordable American food products to dinner tables across the country. In May, Secretary Rollins announced an additional $14.5 million in funding to reimburse states for their meat and poultry inspection programs and called on Congress to more sustainably fund these programs moving forward. This funding is needed to support more than 1,500 American businesses that rely on state inspection, including small and very small meat and poultry processors. Secretary Rollins also signed a Memorandum of Understanding with the National Association of State Departments of Agriculture (NASDA) in May to improve collaboration between USDA and states.
Additionally, in 2025, FSIS signed updated, comprehensive cooperative agreements with all 29 states that operate state meat and poultry programs. These agreements clarify expectations for oversight and enforcement of food safety laws, provide comprehensive training for inspectors, and ensure regular coordination with FSIS. As part of its enhanced oversight of Talmadge-Aiken state cooperative programs, FSIS has completed in-person reviews at 77 percent (320 of 414) of Talmadge-Aiken establishments in the first six months of 2025.
5. Empowering FSIS Inspectors to Take Action to Drive Compliance
FSIS is exercising its enforcement authorities and issuing notices of intended enforcement or suspending operations at establishments to address recurring noncompliance and ensure safe food production. The agency has taken 103 enforcement actions in 2025 to protect consumers, an increase of 36 percent over the same period in 2024. Additionally, FSIS has instructed its field supervisors to conduct in-person, follow-up visits when systemic issues are identified during an FSA. Follow-up visits by FSIS field supervisors bolster oversight to ensure an establishment fully addresses issues identified during an FSA and could inform enforcement action by FSIS.

Secretary Rollins Unveils New USDA Food Safety Policy Plan
The National Resources Defense Council (NRDC) has created a comprehensive map, based on U.S. Environmental Protection Agency (EPA) data, that shows the extent of "forever chemical" contamination in U.S. drinking water—revealing that more than 73 million people are being served by water systems with contamination above now-revoked EPA maximum limits for any one of six problematic per- and polyfluoroalkyl substances (PFAS). In May 2025, EPA announced plans to partially roll back the Biden-era maximum limits, rescinding the regulations for four of the chemicals and delaying the compliance date for the remaining two PFAS types. NRDC experts say their map demonstrates how rolling back these drinking water limits will leave millions of Americans unprotected.
More specifically, in April 2024, EPA issued the first-ever national, legally enforceable drinking water standard to protect Americans from exposure to harmful PFAS: PFOA, PFOS, PFNA, PFHxS, and HFPO-DA (also known as GenX chemicals), as well as a limit for mixtures of any two or more of four PFAS: PFNA, PFHxS, PFBS, and GenX chemicals. These PFAS types are known to cause harm to human health and for their widespread environmental contamination.
On May 14, 2025, Trump-appointed EPA Administrator Lee Zeldin announced that the agency would be rescinding the regulations for PFNA, PFHxS, GenX chemicals, and PFBS. EPA also extended the compliance deadline for PFOA and PFOS from 2029 to 2031.
Exposure to PFAS has been linked to cancer, impacts to the liver and heart, and immune and developmental damage to infants and children. PFAS are often referred to as "forever chemicals" due to their inability to break down in the environment or the human body
According to NRDC's map, which visualizes data from EPA's Fifth Unregulated Contaminant Monitoring Rule (UCMR5) testing program, more than 73 million people are being served by water systems with water that had at least one UCMR5 test sample above the EPA's PFAS threshold. Of those 73 million Americans, 30 million are served by water systems with PFAS concentrations exceeding limits for one of the four PFAS that EPA plans to roll back.
Moreover, when additional, unregulated PFAS are considered, the data shows that nearly half of the people across the U.S. whose water was tested under UCMR5 are supplied by systems that detected some level of PFAS. UCMR5 testing is ongoing, and the number of Americans exposed to high levels of PFAS through their drinking water may increase as additional results are released.

EPA Revokes PFAS Drinking Water Limits, Leaving More Than 30 Million at Risk
A study by North Carolina State University (NC State) researchers has demonstrated a concerning presence of antimicrobial resistance (AMR) genes among Salmonella isolated from retail poultry meat.
AMR is a major threat to global public health, causing a growing number of difficult-to-treat infections in humans, and recognized by the World Health Organization (WHO) as one of the top ten health challenges facing humanity in the 21st century. At present, AMR illnesses cause more than 700,000 deaths annually and are expected to outnumber cancer fatalities by 2050. The excessive use of antibiotics in farmed animals is a leading cause of rising AMR among bacteria, such as Salmonella.
The blaCTX-M-65 gene, an AMR gene with traits that facilitate easy transfer between bacteria, has been increasingly reported in Salmonella in food animals. Therefore, the researchers sought to understand the prevalence of and characterize the blaCTX-M-65 gene in Salmonella enterica isolated from poultry meat at retail.
To do so, the NC State researchers analyzed U.S. National Antimicrobial Resistance Monitoring System (NARMS) data for poultry products sampled from North Carolina retail stores between 2020 and 2024, including 132 S. enterica isolates representing 25 serovars, with S. Infantis (34 isolates) and S. Kentucky being the most prevalent. The isolates were then subjected to antibiotic susceptibility testing.
Of the 132 Salmonella isolates, 14 were multidrug resistant (MDR), which whole genome sequencing (WGS) analysis revealed as belonging to three serovars: S. Infantis (11 isolates), S. Seftenberg (one isolate), and S. I -:r:1,5 (two isolates). All 14 of the isolates carried the blaCTX-M-65 gene, and 13 isolates harbored a quinolone resistance-determining mutation. Genes encoding resistance to aminoglycosides, sulfonamides, and tetracyclines were also identified.
The presence of genes that could provide potential environmental survival advantages were also noted, raising concern about Salmonella persistence in food production environments.
Furthermore, the presence of Sequence Type 32 (ST32) among S. Infantis isolates, an ST frequently associated with this serovar, supports the notion that certain lineages of S. Infantis are adept at acquiring and disseminating AMR genes via mobile genetic elements, such as plasmids. ST32 was also found in the two S. I -:r:1,5 isolates, which may indicate a shared evolutionary pathway between the serovars regarding the acquisition of AMR traits. Previous studies have shown a high prevalence of MDR associated with ST32, suggesting a history of successful gene transfer between bacteria.
Additionally, in the sole S. Seftenberg isolate, blaCTX-M-65 was assigned to ST14, pointing to the adaptability of AMR genes across different Salmonella serovars. "This highlights the importance of continuous surveillance, especially in understudied or less prevalent serovars, to prevent the silent spread of critical resistance genes such as blaCTX-M-65," said the researchers.
Overall, the detection of blaCTX-M-65 across multiple serovars suggests that its spread is not restricted to a single lineage or serovar; rather, it is a widespread phenomenon. The researchers underline the importance of routine surveillance for MDR Salmonella enterica serovars, particularly those harboring blaCTX-M-65, and call for comprehensive strategies, including genomic monitoring and responsible antimicrobial use, to curb the spread of AMR in food production settings.
The study was authored by Daniel F. M. Monte, Ph.D.; Erin Harrell, M.S.; Lyndy Harden; and Siddhartha Thakur, D.V.M., Ph.D. of the Department of Population Health and Pathobiology in NC State's College of Veterinary Medicine.

Study: Spread of Antibiotic Resistance Genes in Salmonella is a Problem for U.S. Poultry
A new peer-reviewed study suggests that half of Escherichia coli O157:H7 illnesses contracted from romaine lettuce consumption are caused by untreated irrigation water applied to crops via overhead spray. The study offers actionable interventions that would be the most impactful in reducing the risk of E. coli contamination of leafy greens.
E. coli is a significant microbial risk to leafy greens safety, having caused seven outbreaks between 2015 and 2021 alone—six of which were multistate—resulting in 4,274 confirmed illnesses, 766 hospitalizations, and 11 deaths. The number of E. coli outbreaks linked to leafy greens in the U.S. has increased between 1996 and 2016, with E. coli O157:H7 emerging as the leading source.
To investigate points of pre- and postharvest E. coli O157:H7 contamination along the fresh-cut romaine lettuce production and consumption chain, the researchers developed a quantitative microbial risk assessment (QMRA) model that accounts for the pathogen's exposure and spread throughout the farm-to-fork continuum. The model was adapted from several different QMRAs for leafy greens and leverages a novel equation at the preharvest stage. The new QMRA has been made publicly available as a decision support tool for managing risk factors of E. coli O157 contamination in the U.S.
The model simulated E. coli O157:H7 counts on fresh-cut romaine from farm to fork, providing insight into the influence of various pre- and postharvest factors on microbial food safety risk. Results showed that 52 percent of romaine lettuce E. coli O157:H7 outbreaks occur due to contaminated, untreated overhead irrigation water. This risk can be reduced by as much as 96 percent through water treatments or by switching to furrow or drip irrigation, which reduce the probability that water directly touches the lettuce leaves, say the researchers.
In postharvest stages, maintaining the romaine cold chain is crucial to preventing high numbers of illnesses during E. coli outbreaks.
However, the model showed that interventions like consumer washing, cattle vaccination, and temperature reduction at retail were considerably less effective at reducing the number of illness cases.
The researchers acknowledged knowledge gaps in their model, including the microbial quality of irrigation waters, as well as the effectiveness of preharvest surface water treatments and postharvest wash processes. Still, the researchers believe their QMRA will be a useful tool in the development of sustainable food safety strategies along the supply chain.
The study was authored by Ece Bulut, Ph.D.; Sarah I. Murphy, Ph.D.; Renata Ivanek, D.V.M.; and Martin Wiedmann, Ph.D. of Cornell University; Laura K. Strawn, Ph.D. with Virginia Tech; and Michelle D. Danyluk, Ph.D. with the University of Florida. Their work was supported by a U.S. Department of Agriculture National Institute of Food and Agriculture (USDA-NIFA) grant.

Half of Romaine Lettuce E. Coli Outbreaks Caused by Overhead Irrigation Water, Study Finds
A new study has highlighted the key challenges that produce growers face when implementing new, on-farm food safety practices, as well as motivators and needs for adoption.
In light of high-profile foodborne illness outbreaks that have occurred in the years since the Produce Safety Rule (PSR) under FSMA went into effect, the study's researchers sought to understand how growers selling to U.S. markets have progressed in the implementation of new microbial risk reduction practices, as well as the challenges and barriers that remain to further adoption of new practices. To gain industry perspectives on the topic, a survey was sent to individuals who participated in Produce Safety Alliance produce safety training in the past. More than 700 responses were included in the dataset, almost half of which (47 percent) reported being covered by the PSR. Approximately one-third of respondents (33 percent) reported being exempt or excluded from the PSR.
Although the majority of respondents reported implementation of produce safety practices on their farms, seven percent reported not implementing risk reduction practices. However, the number of growers implementing such practices continues to increase in comparison to previous surveys. Not only did growers report economic and legal incentives for the adoption of new produce safety practices but also expressed being motivated by a personal commitment to produce safety.
Larger operations and farms subject to third-party audits were more likely to implement produce safety practices. However, farms of all sizes reported financial and time constraints being a challenge to optimal implementation of microbial risk reduction strategies. Given more time and money, growers could more effectively educate themselves about requirements and new practices, afford necessary updates, and prioritize among numerous and conflicting needs.
Evidence-based training, outreach, and support were identified as key needs for growers to implement appropriate produce safety practices. Specifically, the researchers recommended education for challenged growers that simplifies the adoption of relevant risk reduction tools and techniques. Additionally, targeted research focusing on vulnerabilities, behavioral change factors, and cost-effective mitigation strategies could help assist growers in effectively identifying risks and implementing safety practices.
The study was conducted by a team of researchers from USDA, FDA, the University of Vermont Extension's Northeast Center to Advance Food Safety (NECAFS), the Produce Safety Alliance, and the University of Florida, as well as an independent industry expert.

Produce Growers Say Time, Money are Biggest Barriers to Adoption of On-Farm Food Safety Practices
Fish and fishery products have been increasingly implicated in multistate Salmonella outbreaks over the past decade. To better understand the characteristics and contributing factors of these outbreaks, researchers from FDA and CDC reviewed foodborne illness outbreak investigation data from 2012–2021.
Using CDC's National Outbreak Reporting System (NORS); System for Enteric Disease Response, Investigation, and Coordination (SEDRIC); and PulseNet databases, the researchers accessed foodborne illness outbreak investigation records for multistate Salmonella outbreaks linked to fish and fishery products (excluding raw molluscan shellfish). Data included epidemiologic interviews and case information, laboratory testing of clinical and food samples, inspections and environmental sampling, and traceback activities.
Between 2012 and 2021, there were five confirmed multistate Salmonella outbreaks associated with fish and fish fishery products, three of which were linked to frozen tuna, one to frozen shrimp, and one to fresh seafood. In four of the five confirmed outbreaks, the outbreak strain was detected in products imported from India, Indonesia, and Vietnam. The fifth confirmed outbreak had an environmental sample that yielded the outbreak strain.
In addition to the five confirmed Salmonella outbreaks, there were four additional multistate Salmonella outbreaks with fish and fishery products identified as a suspected vehicle of illness, two of which were associated with frozen tuna, one with smoked fish, and one with fresh tuna and salmon. In total, the outbreaks caused 721 cases of illness, with 104 hospitalizations.
Some of the most common contamination factors identified in the outbreak investigation data included the use of contaminated water in manufacturing food and ice; using water hoses that create overspray, which may cross-contaminate food and food contact surfaces in the processing areas; and inadequate cleaning and sanitizing. Additionally, exposure to birds, insects, and other pests in processing environments was noted in some cases, as were improperly sanitized surfaces and cutting tools.
The researchers also noted significant traceback difficulties faced during the outbreak investigations, especially with imported seafood due to fragmented supply chains and poor documentation.
Based on their findings, the researchers underlined the need for improved sanitation practices in seafood processing; better traceability systems, especially for imported products; and stronger collaboration between regulatory agencies and industry to prevent future outbreaks. The study reinforces the importance of environmental controls and food safety education across the seafood supply chain.

10-Year Analysis of Fish Salmonella Outbreaks Underlines Need for Better Import Traceability
Food Standards Australia New Zealand (FSANZ) has received ministerial approval for four changes to the Food Standards Code. It has also released targeted guidance for the seafood and cell-cultured food sectors in the latest update of the Compendium of Microbiological Criteria for Food.
On July 25, 2025, Australian Food Ministers endorsed the following FSANZ Board decisions, clearing the way for their formal adoption into the Food Standards Code:
- Mandatory energy labeling on alcoholic beverages (P1059) requires alcoholic beverage manufacturers to clearly display energy (kilojoule) content labels on each drink
- Changes to carbohydrate and sugar claims on alcoholic beverages (P1049) clarify that nutrition content claims about sugar can be made on alcoholic beverages (but claims about individual sugars, such as fructose, or other components of carbohydrate are prohibited)
- New definitions for genetically modified foods (P1055) will ensure food regulation keeps pace with technology, is science-based, and continues to focus on safety—details about the new definitions can be read here
- A change to allow food to be served in Australian aircraft cabins when pet cats and dogs are present under controlled conditions (A1314) will support more flexible travel options for pet owners, without compromising food safety.
Additionally, FSANZ has also updated the Compendium of Microbiological Criteria for Food to include new chapters providing targeted guidance for the seafood sector and on emerging cell-cultured foods. The compendium is a practical reference for food businesses and regulators, containing nationally consistent microbiological criteria for ready-to-eat (RTE) foods and specific commodities, along with advice on environmental monitoring.
The new seafood chapter provides best practices for managing key microorganisms, microbiological criteria, and process hygiene for RTE products, shellfish, and other seafood.
The new cell-cultured food chapter—developed in consultation with stakeholders through the now-approved application for cell-cultured quail meat—sets out agreed microbiological criteria to support safe production.

FSANZ Announces Changes to Food Standards Code; Provides New Guidance for Seafood, Cell-Cultured Sectors
England saw a 26 percent rise in Shiga toxin-producing Escherichia coli (STEC) infections from 2023 to 2024, according to the latest data from the UK Health Security Agency (UKHSA). The rise in cases may be attributable to one foodborne illness outbreak involving contaminated salad leaves, officials say.
Of the 2,544 laboratory-confirmed STEC cases seen in England in 2024, 564 were STEC O157 and 1,980 were non-O157 serotypes. Hemolytic uremic syndrome (HUS) developed in 2.1 percent of STEC O157 patients and 1.7 percent of non-O157 patients. Among STEC O157 cases, two people died, and among non-O157 cases, five people died. The highest incidence of STEC cases in 2024 was in children between one and four years of age.
In 2024, UKHSA and partner agencies investigated five STEC outbreaks—all of which were non-O157—comprising 467 cases (348 in England specifically). The sources for three of the outbreaks were contaminated beef, fresh fruit, and salad leaves. The largest outbreak was linked to contaminated leafy greens which resulted in 293 cases (196 cases in England). Of the 293 cases, 126 people were hospitalized, 11 developed HUS, and two died.
STEC non-O157 cases in 2024 increased nearly three times since 2019, while O157 cases have returned to pre-COVID-19 pandemic levels. The increase in STEC non-O157 seen in 2024 is due to the outbreak linked to salad leaves. Additionally, more cases of illness are being detected due to the growing use of polymerase chain reaction (PCR) testing technology in laboratories.

STEC Illnesses in England Rose by 26 Percent in 2024, Non-O157 STEC Cases Tripled Since 2019
Researchers have developed a new database that consolidates disparate EU food monitoring data on pesticide residues, veterinary drug residues, and chemical contaminants into a unified, structured dataset that improves accessibility and enables analysis. Using this database of nearly 400 million entries, the researchers identified food safety monitoring trends across Europe between 2000 and 2024.
The new CompreHensive European Food Safety (CHEFS) database draws from official food safety monitoring data that are collected by Member States and submitted to the European Food Safety Authority (EFSA). This data includes more than 392 million analytical results, representing over 15.2 million samples of more than 4,000 different foods.
Analysis of the Data Reveals Food Safety Trends
Using AI analysis of their CHEFS database, the researchers examined trends in food safety monitoring between 2000 and 2024. Of the 392 million analytical results in the CHEFS database, 0.025 percent (97,294) were non-compliant. Sampling strategies represented included random sampling (53.1 percent), risk-based sampling (23.4 percent), suspect sampling (5.3 percent), and "other" sampling (18 percent).
Overall, the number of analytical results increased between 2000 and 2024, except for a dip in 2020 and 2021 attributable to the COVID-19 pandemic. Not only has the number of analytical results grown over time, but the quality of the metadata reported to EFSA has improved; for example, the percentage of samples with an unknown origin decreased tenfold between 2017 and 2024.
The most frequently monitored hazards were toxic heavy metals and mycotoxins in the chemical contaminant hazard category; organophosphates chlorpyrifos, diazinon, and pirimiphos-methyl in the pesticide residues hazard category; and antibiotics erythromycin, danofloxacin, and doxycycline in the veterinary medical product residues (VMPRs) hazard category. Chemical contaminants had the highest rate of non-compliant results, followed by pesticide residues and VMPRs. Even despite the near-total use of selective sampling strategies for VMPRs, the hazard category had the lowest non-compliance rate, suggesting the effective regulation of veterinary drug use for food animals in the EU.
Of the foods most frequently analyzed, milk and dairy products were most often found to be above legal limits in the chemical contaminant group, animal feed was most often above legal limits for pesticides, and cereal and grains were most often above legal limits for VMPR.
The number of analytical results reported to EFSA differed greatly between countries. Germany and France had the highest reporting rates, which are also the second and first most productive food-producing countries in the EU, respectively. On the other hand, in countries like Poland and Spain, analytical results reported to the EU were relatively few, considering their comparatively high food production volumes. Additionally, the analysis showed that contaminated samples more often originated from countries outside of the EU.
The Importance of Traceability for More Useful Data
The researchers underscored the need for better traceability to meaningfully connect the CHEFS database with external datasets, because it is crucial to have accurate metadata on the origin of food products. Sometimes, country of origin is missing from entries in the database or it is unclear whether country of origin refers to the place of production, processing, packaging, import, or even sampling location. Accurate origin information would help identify sources of contamination and enable targeted interventions. Knowing the date of an analytical result could also add great value, making data usable for study of climate conditions on contaminants of crops, for example.
In the future, the researchers envision CHEFS being integrated with external datasets (e.g., climate data, economic and geopolitical indicators, and legislative records) to investigate indirect influences on food safety; for example, associations between changing temperatures and humidity on the presence of toxins or the prevalence of a certain contaminant following a regulatory adjustment to legal limits. Additionally, CHEFS shows promise for integration with comparable food safety monitoring systems from across the globe, enabling cross-regional comparisons and strengthening global food safety surveillance.
Opportunities for AI Applications in Food Safety Research and Policymaking
CHEFS also opens up new opportunities for advanced AI applications in food safety research and policymaking. The Rapid Alert System for Food and Feed (RASFF) is an important source of food safety data in Europe, containing notifications on food and feed that pose a risk to public health. Although the sensitive nature of RASFF data makes it not feasible to include in CHEFS, the researchers see an opportunity to use an AI approach called federated learning to compare CHEFS and RASFF data. Federated learning is a type of machine learning in which models are trained across multiple decentralized devices or servers holding local data, without requiring the exchange of sensitive data.
The CHEFS database can be accessed here.

Database Combines 392 Million EU Food Safety Analytical Results, Reveals Contamination Trends
The Food and Agriculture Organization of the United Nations (FAO) has released a report that provides the first comprehensive global review of the food safety hazards, controls, and regulatory considerations associated with modern indoor farming.
Perceived benefits of indoor farming, or controlled environment agriculture (CEA), include sustainability, adaptability to climate change, and improving food security. In the present day, CEA production of short-term crops such as microgreens and baby leaf, along with mature leafy greens that are traditionally cultivated outdoors, has become commercially viable.
Although it is often assumed that CEA offers more easily managed food safety risks than traditional farming, the publication emphasizes that food safety issues linked to indoor-farmed crops are generally similar to those found in conventional outdoor agriculture. Specifically, microbial hazards exist related to seeds, growth substrates, and water, as well as operations that share features with sprouted seed production. While the hazards are comparable to those in conventional farming, differences in production systems create complexity for risk assessment and management.
While indoor farming eliminates some of the uncontrollable routes of contamination inherent to outdoor farming, outdoor environments also promote some natural pathogen die-off through microbial competition, fluctuating temperatures, and desiccation. The stable conditions in CEA may enable longer pathogen persistence, but indoor systems may also often offer more opportunities for proactive control over inputs such as seeds, water, growth substrates, and fertilizers.
Interestingly, recent research suggests that introducing dynamic environmental conditions within indoor farms may optimize energy use and plant productivity and potentially reduce pathogen persistence.
Microbial hazards are especially relevant to CEA due to the high-humidity, water-based systems that are commonly used. Although microbiological risks associated with CEA are much more well documented than chemical hazards, the report also notes chemical issues such as environmental contamination and contamination from equipment-related materials.
Additionally, the report proposes that, due to the exclusion of wildlife and the ability for enhanced hygienic practices in CEA, indoor farming may more closely resemble sprout production than conventional field agriculture. Despite this, regulatory frameworks have yet to reflect this similarity.
Like sprouted seed production, CEA benefits from tailored interventions, including seed disinfection, irrigation water testing, and hygienic practices to try and promote a beneficial microflora. Additional control points specific to indoor systems may involve the growth substrate, fertilizers, and production environment, which require targeted management to reduce contamination risks.
The report outlines research and knowledge gaps that exist regarding CEA food safety. A critical challenge lies in the heterogeneity of indoor farming systems, which differ in design, input materials, environmental control levels, and operational practices. This diversity complicates the ability to generalize food safety risks and hinders the development of universal risk management strategies. To address this, the authors suggest comprehensive risk assessments to compare food safety risks across various indoor systems, potentially facilitated by global expert consultations. Additionally, artificial intelligence (AI) and predictive modeling offer potential utility for risk characterization, given that quality, standardized data can be collected.
Priority research areas for future work underlined in the report include, but are not limited to: contamination sources in seed production, the prevalence of human pathogens in indoor farming inputs (i.e., seeds, fertilizers, substrates, and pesticides), the behavior of pathogens in CEA systems, input contamination and pre- and postharvest intervention strategies, postharvest pathogen reduction techniques, and strengthening food safety culture.

FAO Report Characterizes Indoor Farming Food Safety Considerations

Kirk Tanner has been appointed President and CEO of The Hershey Company, succeeding Michele Buck, who is retiring.

TANNER
Kroger has named George Vincent as secretary and general counsel.
Duravant's Food Sorting and Handling Group has appointed Dean Ekkaia as Director of Product Management for optical sorting solutions.

EKKAIA
Electrolux Professional Group has appointed Sarah Lavis as Director of Strategic Brand Management.
Peggy Poole became the 86th President for the Institute of Food Technologists (IFT) on September 1, replacing Dr. Christopher Daubert of the University of Missouri. Dr. Gunnar Sigge of Stellenbosch University in South Africa became President-elect. IFT has also hired Eric Ellis as its new Chief Financial Officer.

POOLE
Peak Technologies has appointed Michael Wills as Chief Revenue Officer for North America.
The Safe Quality Food Institute (SQFI), a division of FMI—The Food Industry Association, has appointed Rachel Anderson its new Vice President of business development. FMI—The Food Industry Association has also appointed Jessica Tanner as Director of Legal Operations.

ANDERSON
The International Fresh Produce Association (IFPA) has reorganized to enhance and amplify its goals in the areas of produce safety and food safety regulations. Under the new structure, IFPA's Regulatory and Compliance Expert, Paul Lewis, will join the government relations team and report to Alexis Taylor, Chief Global Policy Officer. Other members of the food safety team, including Angie Fraser, Vice President of Food Safety and Quality; Jorge Quintanilla, Food Safety Manager; and Alison Saltzmann, Food Safety Support Coordinator, will join the science and technology (SciTech) team under Max Teplitski.

Women in Food Safety Celebrates Five Years, Earns Nonprofit Status
Women in Food Safety (WIFS), a community dedicated to empowering and advancing women in the food safety industry, has achieved official designation as a nonprofit organization. This milestone coincides with WIFS's fifth anniversary, marking a new chapter of growth and impact. Founded in 2020, WIFS has evolved into a global network of more than 1,500 professionals, offering mentorship, advocacy, and connection for women at all stages of their careers in food safety. With nonprofit status, WIFS is poised to expand its educational initiatives, strengthen industry partnerships, and deliver new resources that support long-term professional development.
Over the past five years, WIFS has hosted dozens of networking events, launched the Roots to Success scholarship, and introduced the Community app, a platform for women to share their voices and experiences. As a nonprofit, WIFS will further its efforts to:
- Expand mentorship and leadership programs for emerging food safety professionals
- Offer scholarships and career resources to support women entering and advancing in the field
- Strengthen partnerships with universities, companies, and industry associations
- Drive thought leadership on inclusion, food safety culture, and the future of food safety and quality.
WIFS, which can be found at www.womeninfoods.org and on LinkedIn, invites members, partners, and allies to join in shaping the next chapter of its mission to elevate women in food safety.

FDA, Western Growers Sign Data-Sharing MOU in Line With 'Roadmap to Produce Safety'
Western Growers (WG) and FDA have signed a Memorandum of Understanding (MOU) to improve proactive, science-based food safety standards to prevent foodborne illness outbreaks linked to produce. The goal of the collaboration is to create opportunities for FDA and WG to foster a better understanding of safe practices in the growing, harvesting, packing, and holding of fresh produce. The MOU is aligned with FDA's goals in the new Reagan-Udall Foundation's Roadmap to Produce Safety.
The MOU establishes a food safety data-sharing pilot project for fresh produce—including data such as pathogen testing results—using WG's proprietary GreenLink food safety data-sharing platform. The MOU also establishes a broader framework for ongoing data-sharing between WG and FDA. Drawing on successful data-sharing programs in other areas, such as FDA's seafood safety initiative, the effort aims to create a sustainable model that protects public health by advancing food safety knowledge, fostering preventive food safety behaviors, supporting resource management for public health agencies, and minimizing supply chain disruptions.

USDA-FSIS, AOAC Sign MOU for Collaboration on Validation of HACCP Food Safety Systems

USDA's Food Safety and Inspection Service (USDA-FSIS) and AOAC International have signed an MOU, which establishes a strategic framework for developing, validating, and recognizing methods used by FSIS labs and regulated establishments for the verification of HACCP-based food safety systems.
The MOU outlines how AOAC and FSIS will cooperate on work related to mutual interests, including:
- Collaboration on scientific method training and other educational initiatives
- Development of Standard Method Performance Requirements (SMPRs) documents, validation guidance, and/or method validation protocols
- Development of proficiency testing programs
- Adoption or certification of methods for specific regulations or monitoring programs, such as Salmonella quantification.
Portable, Rapid Sensor Can Simultaneously Detect Microbiological, Chemical Food Contaminants

Developed by scientists from the University of Texas at Dallas, EnliSense's READ FWDx—short for Rapid Electroanalytic Diagnostic Food Water Diagnosis—is a novel, compact rapid sensor that can detect microbiological and chemical food contaminants, such as dangerous pathogens, toxic pesticides, and antibiotics. The developers hope their proof-of-concept device will one day be used in both processing facilities and at home to screen for food safety.
READ FWDx's portable design and efficient capabilities are enabled by a technology called adaptive electrochemical impedance spectroscopy. Traditional detection technologies are unable to simultaneously test for microbiological and chemical contaminants. However, READ FWDx combines 16 sensors with different detection capabilities on a single chip, allowing it to measure the concentrations of various contaminants in one sample.
EnLiSense and the research team behind READ FWDx envision delivering the sensor to the public in the form of a single-use, portable chip with a year-long shelf life when stored in the home. They also hope to interest the food industry in READ FWDx as an onsite, point-of-use detection device to ensure food safety when rapid turnaround is required.
Australia Approves First Milk Fat Globule Membrane for Use in Infant Formula

Food Standards Australia New Zealand (FSANZ) has approved Arla Foods Ingredients' application for the use of its milk fat globule membrane (MFGM) as a nutritive ingredient in infant formula products. Following formal acceptance by the Australia and New Zealand Food Ministers Meeting, the approval is now effective, and the company has been granted exclusive commercialization rights. Arla Foods Ingredients' Lacprodan MFGM-10 will be the only MFGM ingredient approved for use in products for infants Australia for a minimum of 15 months. Lacprodan MFGM-10 is also the company's first early life nutrition product to be approved by Australian authorities.
The decision only applies to Australia, where Lacprodan MFGM-10 can now be labeled as "Milk fat globule membrane-enriched whey protein concentrate," but not New Zealand, which recently opted out of the Australia-New Zealand joint infant formula products standard.
bioMérieux Acquires Food Industry Genomics Solutions Provider Neoprospecta

bioMérieux has acquired Neoprospecta, a Brazil-based developer of data and genomics solutions for food industry quality assurance programs and microbiological risk prevention. Founded in 2011, biotechnical company Neoprospecta is dedicated to the development and commercialization of innovative microbiological analysis, based on next-generation DNA sequencing and biocomputational analysis.
Among the various technologies and services in its portfolio, Neoprospecta's Neobiome is a strategic tool for decision-making based on microbiome information. The data and genomics analysis platform enables food industry players to enhance the quality and safety of their production by helping track and control areas of contamination, assess and manage risk, improve processes, and define good practices for microbiological control.
With its acquisition of Neoprospecta, bioMérieux is strengthening its Data and Genomics offering, which comprises innovative pathogen and spoiler investigation tools to prevent contamination, reinforcing the company's Augmented Diagnostics approach.
Ideagen Launches Food and Beverage Division Offering Farm-to-Fork Compliance Solution

By acquiring two leading supply chain softwares—Authenticate and Safefood 360°—and combining them with its own, existing solution (formerly known as Qadex), Ideagen is creating a comprehensive, end-to-end ecosystem for compliance and transparency across the global food industry. The company is also launching a new Food and Beverage Solutions division to meet the demand for solutions that address growing regulatory requirements and the need for sustainable and transparent food systems. The launch underscores Ideagen's dedication to enhancing food safety and sustainability while offering meaningful solutions that address the complex challenges of modern supply chain management.
RESOURCES
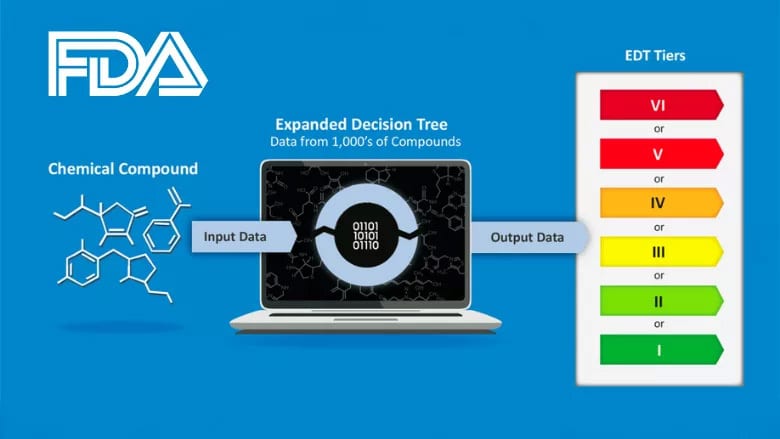
FDA has published its methodology for risk-ranking and prioritizing chemicals in the food supply for systematic post-market safety reevaluation. The new Expanded Decision Tree (EDT) chemical toxicity and risk screening tool provides a consistent, systematic, and science-based approach to support evaluation of the safety of chemicals in food based on their structure and estimated toxicity.
The EDT was submitted for external peer-review in March 2024 and was revised based on input received. It is now available to the scientific community for technical consideration. FDA is developing an automated software solution for the EDT for general public use and will further refine the EDT over time with access to more information about chemicals in the food supply and based on public input.
Expanded Decision Tree: FDA's Food Chemical Toxicity Screening Tool
FDA Releases Decision Tree Tool to Rank Risk of Chemicals in Food for Systematic Safety Evaluation
USDA's Food Safety and Inspection Service (USDA-FSIS) has released a new, generic Hazards Analysis and Critical Control Points (HACCP) model for ready-to-eat (RTE) fermented, salt-cured, and dried products. FSIS previously published the FSIS RTE Fermented, Salt-Cured, and Dried Products Guideline which provides information on FSIS regulatory requirements for the safe production of RTE shelf-stable, fermented, salt-cured, and dried products that rely on multi-hurdle approaches to achieve lethality and shelf-stability. The model illustrates the processing of a dried, fermented sausage in the HACCP processing category of "not heat treated—shelf stable."
Consistent with previous models, the new, generic HACCP model includes a product description, ingredients list, production flow diagram, hazard analysis, and HACCP plan. Flow diagrams in the models demonstrate a general production process and should be modified to reflect the processes used at the establishment. The model is intended to assist establishments in meeting food safety regulatory requirements. FSIS HACCP models may not necessarily apply to all operations or products, and the contents of the models are nonbinding.
USDA-FSIS Publishes New Generic HACCP Model for RTE Fermented, Salt-Cured, Dried Products

The European Commission has published a document to answer frequently asked questions about the requirements of new EU legislation that mandates whole genome sequencing (WGS) testing and data reporting for important foodborne pathogens. Regulation (EU) 2025/179 comes into force on August 23, 2026.
Commission Implementing Regulation (EU) 2025/179 aims to facilitate the swift identification of causes of a foodborne illness outbreak and the related batches, lots, or consignments of potentially unsafe food by requiring WGS analysis and reporting to the European Food Safety Authority (EFSA) for isolates of Salmonella enterica, Campylobacter jejuni and Campylobacter coli, Listeria monocytogenes, and Escherichia coli. When one of these pathogens are suspected to be associated with a foodborne illness outbreak, at least one isolate obtained from animals, feed, food, or the feed/food production environment must be analyzed and data must be submitted to EFSA.
In the case of a multinational outbreak, the competent authority of each Member State where an isolate was detected, and where the isolate is associated or suspected to be associated with an outbreak, are responsible for carrying out WGS. Food businesses must submit isolates to competent authorities for WGS, upon request. The data to be submitted to EFSA along with WGS sequences include reference numbers, pathogen species, and date and Member State of samplings, as well as the description of the food, animal species, feed, or environment from which the isolate was derived. Additionally, laboratories conducting WGS analyses should be ISO 17025-accredited.
The European Commission's Q&A document can be found here.
European Commission Publishes Q&A Document for Mandatory WGS Testing and Reporting



