SCROLL DOWN
Outbreak Investigations of Cyclospora cayetanensis Infections 2013–2020: Progress Made and Challenges Remaining
Recurring outbreaks of cyclosporiasis underscore the need for a comprehensive understanding of how Cyclospora cayetanensis contaminates water and produce
By Stelios Viazis, Ph.D., Fazila K. Shakir, M.H.S., Anne Straily, D.V.M., Adrienne Goodrich-Doctor, Ph.D., Jeffery L. Sumter, Dr.P.H., and Socrates Trujillo, Ph.D.
Video credit: TUNTI/Creatas Video via Getty Images
> REGULATORY REPORT
Cyclospora cayetanensis is a parasitic human pathogen that, when ingested, can cause an intestinal illness called cyclosporiasis, which has been increasing in incidence in the U.S. over the past decade.1,2 While our understanding of this parasite has increased greatly in the past decade, there are still many unanswered questions surrounding this parasite and its recent emergence: How does it make people sick, how does it enter the U.S. food supply, and how are the U.S. Food and Drug Administration (FDA) and its investigative partners responding?
It is helpful to start with what we do know: C. cayetanensis infects the small intestine, causing watery diarrhea with frequent, sometimes explosive, bowel movements. Cyclosporiasis can be treated with trimethoprim-sulfamethoxazole, also known as co-trimoxazole. If not treated, illness may last from a few days to a month or longer. C. cayetanensis is transmitted when feces from an infected individual contaminates food or water; however, it is not transmitted directly from person to person because after being shed, the parasite needs time (estimated one to two weeks, at least) in the environment to become infective.1,3,4 C. cayetanensis is endemic in subtropical and tropical areas worldwide, and people living or traveling in countries where cyclosporiasis is endemic may be at increased risk for infection.5 Due to exposure during their early years, some people from areas where cyclosporiasis is endemic may be infected and shed the parasite without symptoms.
Outbreaks of C. cayetanensis infections have been documented since the mid-1990s in the U.S., Canada, Europe, and Australia, and have been associated with the consumption of fresh produce, including raspberries, basil, mesclun, lettuce, snow peas, cilantro, and green onions.6 The majority of illnesses reported in these non-endemic areas, for which the exposure was identified, were linked to travel or foods imported from endemic areas. Cyclosporiasis is a seasonal illness, and the majority of the cases in the U.S. are illnesses occurring during the months of May through August. Recently, an increase in outbreaks has been seen in non-endemic areas, mostly due to increased international travel, globalization of the food supply, and changes in consumers' dietary habits.5 The increased incidence noted in recent years may also be due partly to changes in diagnostic testing practices in the U.S., such as the increased availability and use of a multiplex molecular assay, which can test for multiple different pathogens simultaneously.7
FDA, in collaboration with the U.S. Centers for Disease Control and Prevention (CDC), as well as state and local public health and regulatory partners, conduct foodborne illness outbreak investigations, including those related to C. cayetanensis infections. Here, the authors review the successes and challenges of identifying and responding to outbreaks caused by C. cayetanensis since 2013, (Table 1) the progress made, the challenges that remain, and what the future holds for addressing such outbreaks.
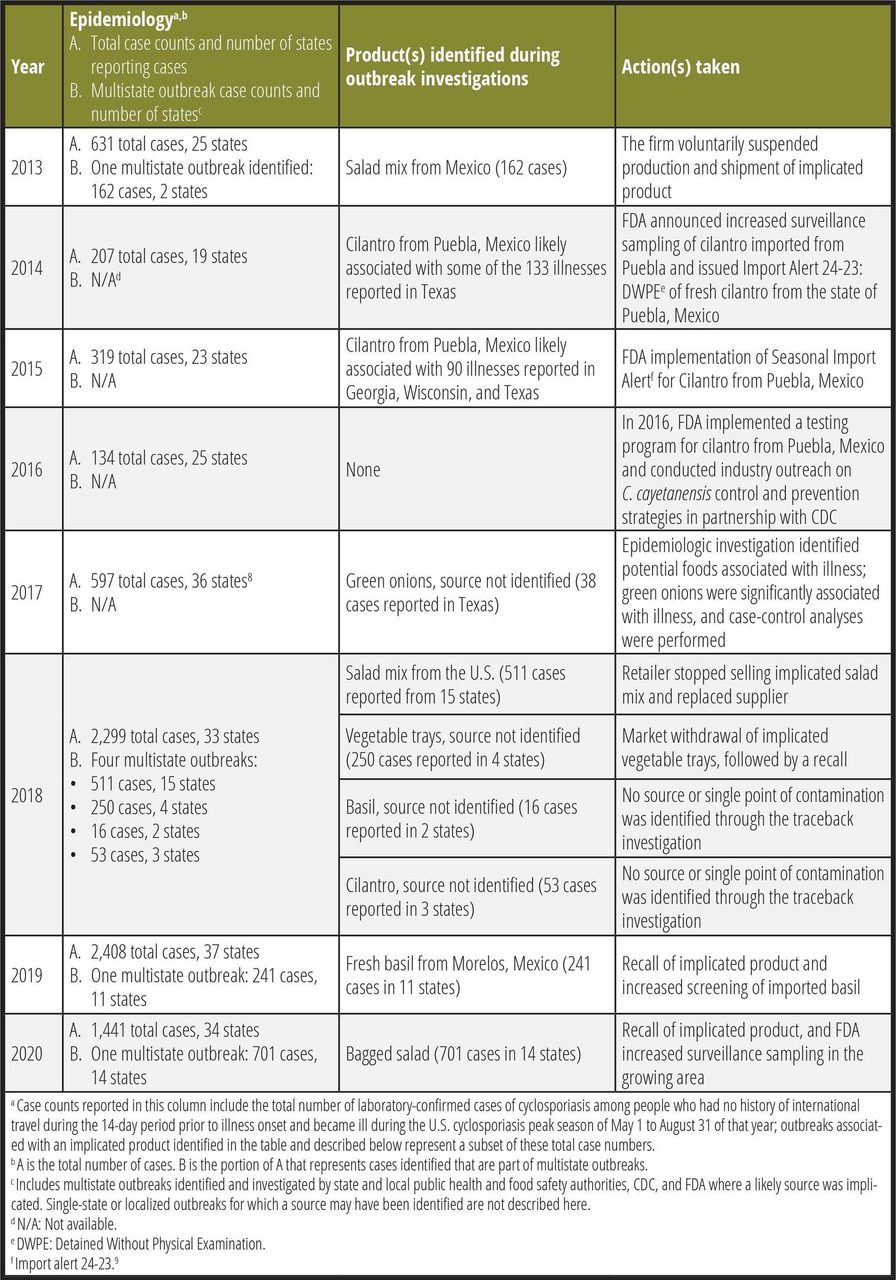
TABLE 1. U.S. Cyclosporiasis Outbreak Investigations, 2013–2020
Prior to 2013, there were outbreaks of cyclosporiasis associated with raspberries and snow peas imported from Guatemala, as well as basil from farms in the U.S. or Mexico.3,10,11 Reports of laboratory-confirmed cases have been increasing in the U.S. in recent years, coinciding with increased use of a molecular diagnostic panel test for gastrointestinal infections that includes Cyclospora, which received FDA clearance in 2014. At present, cyclosporiasis is reportable in 44 states, the District of Columbia, and New York City.12 Since 2013, the U.S. has experienced annual increases of cyclosporiasis incidence. However, in 2020 during the COVID-19 pandemic, FDA observed a decline in reported cyclosporiasis cases, potentially due to travel restrictions imposed or reductions in health care-seeking behaviors that may have resulted in less diagnostic testing for foodborne illness.13
In 2013, epidemiologic and traceback investigations by CDC, FDA, and Iowa and Nebraska state health and agricultural departments identified and linked several illness clusters in those states to a salad mix produced in Mexico, comprising iceberg and romaine lettuce, red cabbage, and carrots.14 The company that produced the salad mix voluntarily suspended production and shipment of any salad mix, leafy green, or salad mix components from its operations in Mexico to the U.S., and resumed production and shipping only after FDA's concurrence. Although salad mix was identified, investigative partners were unable to identify the specific ingredient responsible for the outbreak. Roughly during the same time period, epidemiologic and traceback investigations by public health authorities in Texas, in collaboration with FDA and CDC, indicated that some illnesses among Texas residents were linked to fresh cilantro grown in Puebla, Mexico. As a result of this investigation, FDA implemented targeted surveillance efforts on cilantro products exported to the U.S. from Mexico.15 While some of the illness clusters were traced back to the salad mix and cilantro, the food item(s) that caused the rest of the illnesses during the 2013 season remains unknown.
In 2014, epidemiologic and traceback investigations in Texas focused on four clusters of ill people. People in these clusters reported eating a food item in one of four restaurants containing fresh cilantro in the 14 days before they became ill. A traceback investigation conducted by FDA and Texas indicated that cilantro suppliers from Puebla, Mexico were a source of the cilantro served at the four restaurants.16 The FDA increased surveillance sampling on cilantro imported from firms in the Puebla, Mexico region.
Of the 319 total cases reported in 2015, clusters of illnesses were identified in Texas, Wisconsin, and Georgia. State authorities collaborated with FDA on traceback investigations of these illness clusters. These investigations determined that cilantro from Puebla, Mexico was supplied to restaurants at which people identified in the illness clusters ate, indicating that some illnesses in these states were linked to fresh cilantro from Puebla, Mexico. FDA and Mexican authorities investigated multiple farms and packing houses in Puebla, where they found conditions and practices that may have enabled human fecal pathogens, like C. cayetanensis, to contaminate cilantro and other produce. As a result of these outbreaks and the investigational findings, FDA implemented Import Alert 24-23, which continues to be activated each year from April 1 through August 31. Under Import Alert 24-23, cilantro from growers/packers in Puebla may be detained without physical examination unless the growers/packers have been listed by the FDA on the green list of this import alert, indicating that they have taken steps to control contamination of their cilantro with C. cayetanensis.9
The year 2016 was the first full season that FDA's Import Alert for fresh cilantro from Puebla was in effect and FDA implemented a testing program for cilantro from Puebla, Mexico, as well as industry outreach in Mexico on C. cayetanensis control and prevention strategies. Although 134 cases were still reported in 25 states, these numbers were much lower than any of the cases reported in previous years. Unfortunately, federal and state partners were unable to implicate a food vehicle in the 134 cases.
During 2017, 597 cases were reported in 36 states. In August, the Texas Department of State Health Services and CDC conducted an epidemiologic investigation of a restaurant-associated outbreak of cyclosporiasis within Texas that provided evidence that green onions were the likely vehicle of infection8; however, investigators were unable to trace the green onions to their source.
In 2018, there were two large, multistate outbreaks of cyclosporiasis infections identified.7 One outbreak was linked to a fast-food chain salad mix, and the other was linked to prepackaged vegetable trays. As of September 11, 2018, a total of 511 laboratory-confirmed cases of cyclosporiasis were reported from people who consumed salads from the fast-food chain in 15 states and in New York City. FDA investigated distribution and supplier information for romaine and carrot components of the salad mix, but did not identify a single source or potential point of contamination for this outbreak. On July 26, 2018, FDA recovered C. cayetanensis from an unopened package of a carrot/romaine lettuce salad mix that had been distributed to the fast-food chain. As a result, the fast-food chain decided to voluntarily stop selling salads at impacted restaurants in 14 states and replaced the supplier of salads in those states.
The other 2018 outbreak was associated with prepackaged vegetable trays. As of September 6, 2018, there were a total of 250 laboratory-confirmed cases of cyclosporiasis reported in people who consumed prepackaged vegetable trays from four states. On June 8, 2018, the manufacturing firm withdrew its 6-oz and 12-oz vegetable trays that contained broccoli, cauliflower, carrots, and dill dip from retail market locations. On June 15, it recalled 28-oz vegetable trays distributed to Illinois and Indiana, which included broccoli, cauliflower, carrots, celery, and dill dip. FDA evaluated and reviewed the distribution and supplier information for each component of the recalled vegetable trays as part of the traceback investigation. However, the investigation did not identify a single source or potential point of contamination for any of the items in the recalled vegetable trays.
In 2019, there were 241 laboratory-confirmed cases of cyclosporiasis from 11 states associated with a fresh basil outbreak, accounting for an estimated 10% of the total cases reported during 2019 (n = 2,408).17 FDA conducted a traceback investigation that confirmed that the fresh basil available at points of sale where some consumers became ill was exported to the U.S. by a firm in Morelos, Mexico. FDA increased testing on imported basil to detect and decrease the number of adulterated products entering the U.S. from Mexico. The firm voluntarily recalled the product in response to the outbreak.
Produce safety outreach and education efforts are one of the components of the U.S.–Mexico food safety prevention strategy for fresh produce. In 2020, FDA signed a new alliance with Mexican authorities, creating a new Food Safety Partnership (FSP) to oversee training and outreach activities designed for Mexican produce growers. The FSP's core elements include tech-enabled traceability, smarter tools and approaches for prevention and outbreak response, new business models and business modernization, food safety culture, and the promotion of increased data-sharing to improve outbreak response communications.18
In 2020, a large, multistate outbreak was identified and associated with consumption of a bagged salad mix produced by a large processor under its brand and private labels associated with multiple, large grocery store chains.19 This outbreak accounted for 701 laboratory-confirmed cases of cyclosporiasis from 14 states, which is 48% of the total cases reported during the 2020 season (n = 1,441). FDA-led traceback investigations identified that both the brand name and private labels were produced by the same processing facility. On June 27, 2020, the processor recalled certain production dates for products containing iceberg lettuce, red cabbage, or carrots. FDA conducted a traceback investigation leading to the identification of several farms in the U.S. that may have provided product used in the bagged salad mixes. This marks the first outbreak of C. cayetanensis in which FDA identified domestically based farms through traceback as the sole potential source of implicated product. FDA investigated multiple farms identified in the traceback, but could not conclusively determine the source of this outbreak.
"Ongoing development of laboratory methods for genomic analysis of C. cayetanensis will allow investigators to link illnesses to one another and to potential sources."


“Like many other sectors, the meat and poultry industry has faced unprecedented disruption from the COVID-19 pandemic.”


Common Challenges Encountered During Outbreaks of Cyclosporiasis
Outbreak investigations of cyclosporiasis are largely based on epidemiologic data and traceback investigations. A lack of genetic methods to link cases to one another or to food or environmental samples, like those available for bacterial foodborne pathogens, makes determining the source of the contamination particularly challenging. However, ongoing development of laboratory methods for genomic analysis of C. cayetanensis will allow investigators to link illnesses to one another and to potential sources.
Laboratory Challenges
Outbreaks of cyclosporiasis pose challenges that differ from those caused by bacterial pathogens. With most common foodborne bacterial pathogens, such as Escherichia coli O157:H7, Salmonella spp., and Listeria monocytogenes, investigators are able to use laboratory analyses of the genetic material (i.e., whole genome sequencing, or WGS) to determine whether clinical isolates are closely related to one another and/or to isolates recovered from food or environmental samples. This helps link cases to identified clusters and to confirm hypotheses about the food that was contaminated and that caused the illnesses.
WGS is impractical for routine use in cyclosporiasis outbreak investigations for a number of reasons. One reason is that the C. cayetanensis genome is approximately tenfold larger than a bacterial genome; rarely is enough quality DNA obtained from clinical stool specimens or produce samples to sequence the entire parasite genome. Also, unlike bacterial pathogens, this parasite cannot be propagated in the laboratory. The sexual reproduction cycle of C. cayetanensis, which occurs in the human gastrointestinal tract, poses an additional challenge as it means that no two infections will be genetically identical, even if acquired from the same source.
To address the challenges regarding food and environmental detection of this parasite, FDA has developed multi-laboratory validated methodologies that detect intact oocysts of C. cayetanensis on food matrices such as basil, cilantro, and romaine lettuce, and in agricultural water.20,21 Although current laboratory methodologies do not indicate if the intact oocysts detected are viable, it is a step forward in evaluating the presence of the parasite on food and in the environment, and it adds to the weight of the epidemiologic and traceback evidence.
Epidemiologic Challenges
Outbreaks are defined as two or more ill people who do not live in the same household and who share a common food exposure during the two weeks prior to illness onset. Due to the lack of genetic typing methods, outbreaks of cyclosporiasis are identified epidemiologically—for example, when state health departments receive multiple reports of ill people reporting the same exposures (e.g., same retail location) within a short time frame.
Identifying the food or ingredient responsible for an outbreak of cyclosporiasis can be very challenging. At home and at restaurants, it can be common for produce to be served as a garnish or topping, or for many different types of produce to be mixed together in a single dish, which makes identification and recollection of specific produce items difficult for ill people. Symptoms may begin within two to 14 days of consuming contaminated food and may persist for weeks. The interval between illness onset and report to public health is dependent upon when ill people decide to seek care and are appropriately diagnosed—and diagnosis of this disease can be difficult. As a result, there is generally a lag period of several weeks between when people may have eaten the contaminated food and the case interview, which likely affects a person's ability to accurately remember consumption of specific foods.
A significant number of "sporadic" cases of cyclosporiasis are reported each season that cannot be linked to an outbreak (i.e., because they do not report an exposure consistent with an identified outbreak). These sporadic cases are difficult to interpret and, ultimately, the source of the infection may not be identified.
Traceback Challenges
The complexities that investigators face during traceback investigations stem from the laboratory and epidemiologic challenges described above, in addition to the typical challenges encountered during outbreaks linked to produce.22,23 The lack of validated metagenomic tools (i.e., the study of a collection of genetic material from a mixed community of organisms) for C. cayetanensis limits the ability of investigators to determine whether ill people are infected by the same parasite from a common source, which further limits epidemiologists' ability to identify clusters of illnesses.
Identifying the source of contamination can be challenging for C. cayetanensis, as this parasite requires time in the environment for oocyst sporulation prior to becoming infective. This parasite can also persist in the environment, meaning the contamination event could have taken place weeks or months prior, but the oocysts still have the potential to cause illness if consumed. Evidence of contamination may also no longer be readily apparent when an outbreak investigation is conducted at the identified farm(s) or facilities. Limitations on laboratory methods also make it challenging to link positive food or environmental sample findings to cases. C. cayetanensis is resistant to most common disinfectants used in the food industry and, therefore, also poses a challenge across the food supply to prevent and eliminate contamination.1 This is a significant challenge, particularly for produce growers. Controlling sources of contamination is key to preventing illnesses, as outlined in FDA's "Cyclosporiasis and Produce Fact Sheet."24
"Sampling of product and environmental samples is a critical tool used by FDA for prevention and outbreak response activities associated with foodborne illnesses including C. cayetanensis."


Successes and Breakthroughs
In 2014, FDA established a Foodborne Parasitology Research Program. In collaboration with CDC, FDA has been sequencing the genomes of several different strains of C. cayetanensis, working toward the development of genetic typing methods.25 In 2015, FDA set up a multi-disciplinary workgroup to prioritize the development, validation, and implementation of a method for detecting C. cayetanensis in produce. Subsequently, in 2018, FDA started using the newly validated C. cayetanensis analysis method for produce and was able to recover C. cayetanensis from food samples, which also assisted in guiding some of the traceback activities associated with cyclosporiasis outbreaks that occurred that year. The availability of this method is a significant improvement in FDA's ability to investigate outbreaks of cyclosporiasis and identify the parasite in foods.4,26 In July 2019, FDA developed a novel, validated method to test agricultural water for the parasite.27 In addition, FDA has been engaged in education and outreach efforts focused on produce growers to increase their awareness of C. cayetanensis and actions that can be taken on the farm to reduce the potential of contamination. Some of these actions include proper use, maintenance, and cleaning of toilet and handwashing facilities.24
Since 2014, CDC has been working to develop a genotyping tool that could identify genetically related clusters of cyclosporiasis, which are likely to share a common source. CDC's genotyping method is based on targeted deep amplicon sequencing of eight genetic markers, combined with an ensemble of bioinformatics algorithms to identify genetically related clusters of illnesses. This method was applied to stool specimens collected from cyclosporiasis case patients in 201828 and 201929 and was found to have excellent concordance with epidemiologically identified outbreaks, demonstrating its potential to support outbreak investigations. In 2020, the genotyping method was applied in near-real-time and again demonstrated good concordance with epidemiologically defined outbreaks and complemented epidemiologic investigations.12 In 2022, FDA will begin to apply the CDC genotyping method, with modifications, to C. cayetanensis collected from produce and water samples, which will enable the linkage of human illness to suspect food items—something never before achieved with C. cayetanensis. Although early data indicates that the procedure can be of use, more testing is needed, as well as proper validation prior to its use by FDA.
A Focus on Sampling Findings
Sampling of product and environmental samples (i.e., water) is a critical tool used by FDA for prevention and outbreak response activities associated with foodborne illnesses including C. cayetanensis. Since cilantro and basil are identified, year after year, as the most likely vehicles in outbreaks of cyclosporiasis, FDA has increased seasonal surveillance sampling of these imported fresh herbs from Mexico and other endemic countries. The samples collected are analyzed for human fecal pathogens including C. cayetanensis; if the product sample is found to contain such pathogens, then entry of the product into the U.S. is refused.
FDA also conducts increased surveillance of imported produce, when warranted due to CDC activities or outbreak responses, to gain additional information related to potentially noncompliant firms and adulterated products. As part of this sampling activity, FDA has detected C. cayetanensis in samples of imported blackberries, basil, and cilantro from Mexico, as well as basil from Colombia. Interestingly, while the general seasonality of cyclosporiasis cases in the U.S. runs from May through August, C. cayetanensis was detected in domestically grown cilantro and basil in September 2019. Similarly, FDA detected C. cayetanensis in imported blackberries from Mexico in October 2018. In addition, FDA detected C. cayetanensis in herbs from Mexico in September and October 2018, February 2019, and from Colombia in April 2019.30 These findings suggest that oocysts may be present year-round in produce outside of the typical seasonality of cyclosporiasis cases in the U.S. It is important to note that while oocysts were detected, it does not mean that the oocysts were sporulated/infective or that the produce analyzed would have caused illness if consumed.
In 2017, FDA began a fresh herb surveillance sampling assignment to determine the prevalence of Salmonella spp. and Shiga toxin-producing E. coli in domestic and imported cilantro, parsley, and basil. In 2018, FDA added testing for C. cayetanensis for these herbs. In the preliminary findings, C. cayetanensis was detected in 18 samples, which was approximately 1.4% of the samples analyzed under the assignment. In total, the 18 positive samples consisted of four domestic and 14 imported products associated with basil and cilantro.31 These findings led to 13 firms being placed on import alert and three recalls.
FDA has also sampled and tested domestically grown romaine for C. cayetanensis. For example, in 2018 FDA collected romaine lettuce samples from California to test for C. cayetanensis as part of an investigation related to a fast-food chain salad mix. Four samples of harvested product, corresponding to each of four harvest crews identified in the traceback, were tested. Two out of those four samples yielded C. cayetanensis. All four samples were taken from the same field—a field that was not linked to this outbreak. Two preharvest romaine samples were also analyzed and were negative for C. cayetanensis. While acknowledging the limitations of these results due to the small sample size, these results suggest that workers and/or harvest activities may contribute to contamination of produce.
In response to the 2020 outbreak of cyclosporiasis associated with bagged salad mix, FDA investigated two farms identified through traceback to determine the source and cause of the contamination. As part of the investigations, FDA analyzed water samples from two public access points along a regional water management canal located west of Port St. Lucie, Florida. These samples tested positive for C. cayetanensis. FDA also conducted follow-up investigations of this area to document conditions that may have led to the contamination. During the follow-up, FDA collected additional creek and canal samples and found C. cayetanensis contamination in eight out of nine samples collected across nine locations. Ultimately, FDA was unable to determine if the C. cayetanensis detected in the canal was a genetic match to the clinical cases and, as a result, could not conclusively determine the cause of this outbreak. This marked the first outbreak where the FDA, based on epidemiologic data and traceback information, was able to sample and test water samples for C. cayetanensis using the newly validated FDA method.
Addressing Future Outbreaks
Recurring outbreaks of cyclosporiasis, as well as sample findings in the last decade, underscore the need for a comprehensive understanding of how this parasite contaminates water and produce. As knowledge is gained about this parasite, food safety agencies will need to continue to work closely with industry, environmental agencies, and public health agencies to stop the cycle of contamination. Equally important is the development of practical prevention and control measures that can be applied across the food supply chain.
While additional prevention and control measures are being researched and developed, basic handwashing and hygiene remain critically important in preventing the spread of cyclosporiasis. FDA recently published the "Cyclospora Prevention, Response, and Research Action Plan," which outlines actions necessary to improve prevention, enhance response activities, and fill knowledge gaps to help prevent Cyclospora contamination of foods and prepare for responding to future outbreaks.32 The Cyclospora Prevention Plan includes activities led by FDA, as well as efforts implemented in collaboration with academia, industry, and other partners, such as a charge presented to the National Advisory Committee for the Microbiological Criteria for Food on preventing C. cayetanensis contamination in food.33
With these collaborative efforts in place, there is great potential for federal, state, and local public health partners to be better prepared to respond to outbreaks of cyclosporiasis infections and take the appropriate steps to prevent potential future outbreaks.
Acknowledgements
The response efforts to the outbreaks discussed in this article included public health officials at local and state agriculture and health departments in the U.S., who serve as the backbone of multistate outbreak investigations. The assistance of state partners, including Rapid Response Teams, was crucial in the collection and analysis of product samples, traceback documents, and epidemiologic information.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the U.S. Centers for Disease Control and Prevention and the U.S. Food and Drug Administration.
References
- Ortega, Y.R. and R. Sanchez. "Update on Cyclospora cayetanensis, a food-borne and waterborne parasite." Clinical Microbiology Reviews 23 (2010): 218–234.
- U.S. Centers for Disease Control and Prevention. "U.S. Foodborne Outbreaks of Cyclosporiasis—2000–2017." 2021. https://www.cdc.gov/parasites/cyclosporiasis/outbreaks/foodborneoutbreaks.html.
- Herwaldt, B.L. "Cyclospora cayetanensis: A review, focusing on the outbreaks of cyclosporiasis in the 1990s." Clinical Infectious Diseases 31 (2000): 1040–1057.
- Murphy, H.R., H.N. Cinar, G. Gopinath, et al. "Interlaboratory validation of an improved method for detection of Cyclospora cayetanensis in produce using a real-time PCR assay". Food Microbiology 69 (2018): 170–178.
- Almeria, S., H.N. Cinar, and J.P. Dubey. "Cyclospora cayetanensis and Cyclosporiasis: An Update". Microorganisms 7 (2019).
- Hadjilouka, A. and D. Tsaltas. "Cyclospora cayetanensis—Major Outbreaks from Ready to Eat Fresh Fruits and Vegetables." Foods 9 (2020).
- Casillas, S.M., C. Bennett, and A. Straily. "Notes from the Field: Multiple Cyclosporiasis Outbreaks—United States, 2018". Morbidity and Mortality Weekly Report 67 (2018): 1101–1102.
- Keaton, A.A., N.B. Hall, R.J. Chancey, et al. "Notes from the Field: Cyclosporiasis Cases Associated with Dining at a Mediterranean-Style Restaurant Chain—Texas, 2017." Morbidity and Mortality Weekly Report 67 (2018): 609–610.
- U.S. Food and Drug Administration. 2021. Import Alert 24-23. https://www.accessdata.fda.gov/cms_ia/importalert_1148.html.
- Herwaldt, B.L. and M.L. Ackers. "An outbreak in 1996 of cyclosporiasis associated with imported raspberries." New England Journal of Medicine 336 (1997): 1548–1556.
- Lopez, A.S., D.R. Dodson, M.J. Arrowood, et al. "Outbreak of cyclosporiasis associated with basil in Missouri in 1999". Clinical Infectious Diseases 32 (2001): 1010–1017.
- Barratt, J., L. Ahart, M. Rice, K. Houghton, T. Richins, V. Cama, M. Arrowood, Y. Qvarnstrom, and A. Straily. "Genotyping Cyclospora cayetanensis from multiple outbreak clusters with an emphasis on a cluster linked to bagged salad mix—United States, 2020." Journal of Infectious Diseases (2021).
- Ray, L.C., J.P. Collins, P.M. Griffin, et al. "Decreased Incidence of Infections Caused by Pathogens Transmitted Commonly Through Food During the COVID-19 Pandemic—Foodborne Diseases Active Surveillance Network, 10 U.S. Sites, 2017–2020." Morbidity and Mortality Weekly Report 70 (2021): 1332–1336.
- Buss, B.F., M.V. Joshi, J.L. Dement, V. Cantu, and T.J. Safranek. "Multistate product traceforward investigation to link imported romaine lettuce to a US cyclosporiasis outbreak—Nebraska, Texas, and Florida, June–August 2013." Epidemiology and Infection 144 (2016): 2709–2718.
- U.S. Food and Drug Administration. 2013. FDA Investigates 2013 Multistate Outbreak of Cyclosporiasis. http://wayback.archive-it.org/7993/20171114154925/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm361637.htm.
- U.S. Food and Drug Administration. 2014. FDA Investigates 2014 Outbreak of Cyclosporiasis. http://wayback.archive-it.org/7993/20171114154910/https://www.fda.gov/Food/RecallsOutbreaksEmergencies/Outbreaks/ucm411990.htm.
- U.S. Food and Drug Administration. 2019. Outbreak Investigation of Cyclospora: Imported Fresh Basil (July 2019). https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-cyclospora-imported-fresh-basil-july-2019.
- U.S. Food and Drug Administration. 2021. FDA-SENASICA-Cofepris Food Safety Partnership. https://www.fda.gov/food/international-cooperation-food-safety/fda-senasica-cofepris-food-safety-partnership.
- U.S. food and Drug Administration. 2020. Outbreak Investigation of Cyclospora: Bagged Salads (June 2020). https://www.fda.gov/food/outbreaks-foodborne-illness/outbreak-investigation-cyclospora-bagged-salads-june-2020.
- U.S. Food and Drug Administration. 2019. Dead-End Ultrafiltration (DEUF) for the Detection of Cyclospora cayetanensis from Agricultural Water. https://www.fda.gov/media/131515/download.
- U.S. Food and Drug Administration. 2021. BAM Chapter 19b: Molecular Detection of Cyclospora cayetanensis in Fresh Produce Using Real-Time PCR. https://www.fda.gov/food/laboratory-methods-food/bam-chapter-19b-molecular-detection-cyclospora-cayetanensis-fresh-produce-using-real-time-pcr.
- Irvin, K., S. Viazis, A. Fields, S. Seelman, K. Blickenstaff, E. Gee, M. Wise, K. Marshall, L. Gieraltowski, and S. Harris. "An Overview of Traceback Investigations and Three Case Studies of Recent Outbreaks of Escherichia coli O157:H7 Infections Linked to Romaine Lettuce." Journal of Food Protection (2021).
- Marshall, K., A. Hexemer, S. Seelman, et al. "Lessons Learned from a Decade of Investigations of Shiga Toxin-Producing Escherichia coli Outbreaks Linked to Leafy Greens, United States and Canada." Emerging Infectious Disease Journal 26 (2020): 2319.
- U.S. Food and Drug Administration. 2018. FDA Fact Sheet: Cyclosporiasis and Fresh Produce. https://www.fda.gov/media/123995/download.
- U.S. Food and Drug Administration. 2020. FDA Arming Itself with Science to Help Prevent Cyclospora Infections. https://www.fda.gov/news-events/fda-voices/fda-arming-itself-science-help-prevent-cyclospora-infections.
- U.S. Food and Drug Administration. 2018. New Testing Method Developed by FDA Detects Cyclospora in Salad Mix. https://www.fda.gov/food/conversations-experts-food-topics/new-testing-method-developed-fda-detects-cyclospora-salad-mix.
- Durigan, M., H.R. Murphy, and A.J. da Silva. "Dead-End Ultrafiltration and DNA-Based Methods for Detection of Cyclospora cayetanensis in Agricultural Water." Applied and Environmental Microbiology 86 (2020).
- Nascimento, F.S., J. Barratt, K. Houghton, et al. "Evaluation of an ensemble-based distance statistic for clustering MLST datasets using epidemiologically defined clusters of cyclosporiasis." Epidemiology and Infection (2020): 148–e172.
- Barratt, J., K. Houghton, T. Richins, et al. "Investigation of US Cyclospora cayetanensis outbreaks in 2019 and evaluation of an improved Cyclospora genotyping system against 2019 cyclosporiasis outbreak clusters." Epidemiology and Infection (2021): 1–39.
- U.S. Food and Drug Administration. 2021. FDA Sampling Fresh Herbs, Guacamole and Processed Avocado. https://www.fda.gov/food/cfsan-constituent-updates/fda-sampling-fresh-herbs-guacamole-and-processed-avocado.
- U.S. Food and Drug Administration. 2021. Microbiological Surveillance Sampling: FY18-21 Fresh Herbs (Cilantro, Basil & Parsley) and Processed Avocado and Guacamole Assignments. https://www.fda.gov/food/sampling-protect-food-supply/microbiological-surveillance-sampling-fy18-21-fresh-herbs-cilantro-basil-parsley-and-processed.
- U.S. Food and Drug Administration. 2021. FDA Releases Cyclospora Prevention, Response and Research Action Plan. https://www.fda.gov/news-events/press-announcements/fda-releases-cyclospora-prevention-response-and-research-action-plan.
- U.S. Department of Agriculture Food Safety and Inspection Service. 2021. 2021–2023 National Advisory Committee on Microbiological Criteria For Foods. https://www.fsis.usda.gov/policy/advisory-committees/national-advisory-committee-microbiological-criteria-foods-nacmcf/2021.
Stelios Viazis, Ph.D., is a Consumer Safety Officer, a member of FDA's Coordinated Outbreak Response and Evaluation (CORE) Network, and a part of the CORE Outbreak Analytics team. He focuses on analyzing and communicating outbreak data, lessons learned, and food safety prevention needs through publications, presentations, and guidance documents. Previously, he served as a member of CORE Response, where he coordinated outbreak response efforts, as well as a member of FDA Produce Safety Network, where he served as a Western Region subject matter expert on the Produce Safety Rule.
Fazila K. Shakir, M.H.S., is a Senior Regulatory Health Advisor in the Office of Food Safety, Division of Produce Safety, at FDA's Center for Food Safety and Applied Nutrition (CFSAN). In this role, she has led the development of policies, regulations, and guidance related to the safety of fresh produce, including sprouts, under the Food Safety Modernization Act (FSMA).
Anne Straily, D.V.M., is a Veterinary Epidemiologist with the Parasitic Diseases Branch (PDB) at the U.S. Centers for Disease Control and Prevention (CDC), where she serves as the lead epidemiologist for cyclosporiasis surveillance, outbreak investigation, and response activities for PDB. In addition to her work on cyclosporiasis, she also assists with surveillance, field studies, and technical support for epidemiologic investigations of soil-transmitted helminths, schistosomiasis, toxoplasmosis, and other parasitic diseases, and provides consultation for healthcare providers and public health personnel regarding parasitic diseases.
Adrienne Goodrich-Doctor, Ph.D., is a Commander in the U.S. Public Health Service Commissioned Corps and serves as a Program Coordinator within CFSAN/Office of Compliance within FDA. In this role, she leads development and update of CFSAN compliance programs and field assignments, which provide instructions for the conduct of product sampling, inspections, investigations, and other activities to implement and ensure compliance with federal laws and regulations with specific focus on the Produce Safety Rule.
Jeffery L. Sumter, Dr.P.H., is a Senior Regulatory Operations Officer in the Programs Assignments and Monitoring Branch, Division of Field Programs and Guidance at CFSAN. In this role, he oversees and provides expertise for four food compliance programs, analyzes food safety prevention efforts, and has led the development and response for multistate foodborne outbreak investigations. Concurrently, he serves as a Lieutenant Commander in the Commissioned Corps of the U.S. Public Health Service. He is also an adjunct Professor in the College of Human Environmental Sciences at the University of Alabama.
Socrates Trujillo, Ph.D., is the Chief of the Virulence Mechanisms Branch, Division of Virulence Assessment, Office of Applied Research of Safety Assessment (OARSA). As Branch Chief, he provides guidance and leadership on the planning, coordination, and development of manuscripts on research projects and programs in produce safety, as well as during the management of a laboratory environment. Previous to joining OARSA, Dr. Trujillo was an original member of the FDA Produce Safety Network, an International Program Manager, and a subject matter expert on produce safety.
